
Winter 2014 - Vol. 9, No. 4
Screening for Hereditary Colon Cancer:
A Review from the Genetic Counselor’s Perspective
Erin Sutcliffe, MS, CGC
Certified Genetic Counselor
Cancer Risk Evaluation Program
INTRODUCTION
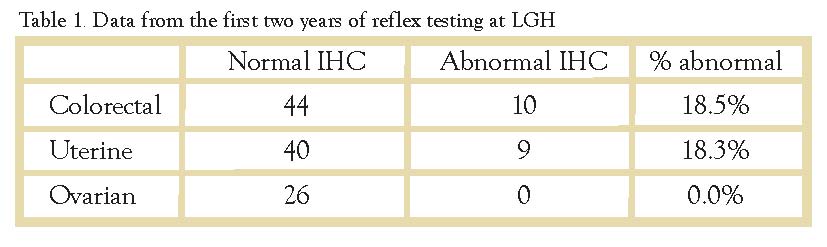
Errors in base pair matching that occur during DNA replication, or as a result of acquired damage, are identified and corrected by the DNA mismatch repair (MMR) genes. Defective mismatch repair has numerous consequences, and can ultimately lead to the development of malignant tumors. Lynch Syndrome, also known as Hereditary Non-Polyposis Colorectal Cancer (HNPCC), is most commonly caused by germline mutations in four of these MMR genes (MLH1, MSH2, MSH6, and PMS2), and is characterized by an increased risk for colorectal, uterine, ovarian, and other cancers (Table 1).

Patients with Lynch Syndrome may have as much as an 80% risk of developing colorectal cancer and up to a 60% risk of endometrial cancer; in the general population these risks are 5-6% and 2-3% respectively. As a result, about 2 – 3% of all colorectal cancers are related to Lynch Syndrome.
According to the National Comprehensive Cancer Network (NCCN), a not-for-profit alliance of 25 of the world’s leading cancer centers, the average age at onset of cancer is approximately 45 - 60 years.1 MLH1 and MSH2 mutations are four times more frequent than mutations in MSH6 or PMS2, which have been associated with lower cancer risks and a later age at onset than is typically seen with MLH1 and MSH2.1
Families with Lynch Syndrome often have multiple members with early-onset colorectal cancer and/or uterine cancer. These patients require more frequent GI screening and gynecologic surgical risk reduction to help manage the elevated risks (Figures 1 and 2).

Deficiency of the mismatch repair genes leads to errors in DNA replication causing repetitive microsatellite sequences, which is also termed microsatellite instability (MSI). Analyzing colorectal and other tumors for the presence of MSI can identify patients who may have Lynch Syndrome. The presence of MSI in the tumor is, however, not diagnostic of Lynch Syndrome because MSI can also occur sporadically.
MICROSATELLITE INSTABILITY (MSI) TESTING
MSI is traditionally assayed by PCR (polymerase chain reaction) in which a standardized panel of five microsatellite markers are sequenced. If at least 2 of the 5 markers are positive, the tumor is reported as MSI-High; one out of 5 markers is termed MSI-Low. For the purposes of screening for Lynch Syndrome, we are most interested in MSI-H tumors. Around 15% of all colorectal tumors are MSI-H. Of these tumors 3% are due to Lynch Syndrome (LS) associated with an inherited mutation in one of the MMR genes.2 The other 12% are related to epigenetic silencing of the MLH1 gene through promoter hypermethylation in the tumor itself, which is not hereditary.2 In 1997, The National Cancer Institute in Bethesda, MD held a workshop in which the criteria for MSI testing were defined.3 These criteria, known as the Bethesda Guidelines, were later revised in 2002.4
MSI testing is recommended in colorectal cancer (CRC) patients who:
- Are diagnosed prior to age 50;
- Are diagnosed with synchronous or metachronous CRC/Lynch cancers;
- Are diagnosed prior to age 60 with MSI-H histologic features such as tumor infiltrating lymphocytes, Crohn’s-like reaction, signet-ring differentiation, or medullary growth pattern;
- Have a family history of CRC/Lynch cancer in at least one first degree relative diagnosed prior to age 50; Have a family history of CRC/Lynch cancer in at least two first/second degree relatives regardless of age.
CLINICAL SIGNIFICANCE OF MSI
In addition to its use as a screening tool for Lynch Syndrome, MSI status plays a role in planning treatment for some patients with CRC. Patients with MSI positive CRC have a better prognosis but will not respond to 5-fluoro-uracil (5-FU) chemotherapy.5,6 So for those patients who may not require adjuvant chemotherapy (i.e. stage II CRC patients), MSI analysis can be an important tool in the risk/benefit discussion of chemotherapy.
IMMUNOHISTOCHEMISTRY (IHC)
Another method commonly used to test indirectly for MSI involves immunohistochemical (IHC) staining of the MMR gene proteins. Absence of staining of one or more of the MMR gene proteins in the tumor is consistent with a mutation in the gene and suggests the presence of MSI. The sensitivity of IHC in predicting MSI is about 92 - 97%.7-9 Approximately 15 - 20% of all CRC will have abnormal IHC.2,10
During the mismatch repair process, the MMR gene proteins form heterodimers and work in complex with other proteins: MLH1 pairs with PMS2, and MSH2 pairs with MSH6. PMS2 is ‘dependent’ on MLH1, and MSH6 is ‘dependent’ on MSH2 such that if you have loss of MLH1 or MSH2, you would also have loss of PMS2 or MSH6, respectively.
It is very unusual to see loss of MLH1 or MSH2 alone, and as a result there are five typical results from IHC testing. (Table 2)
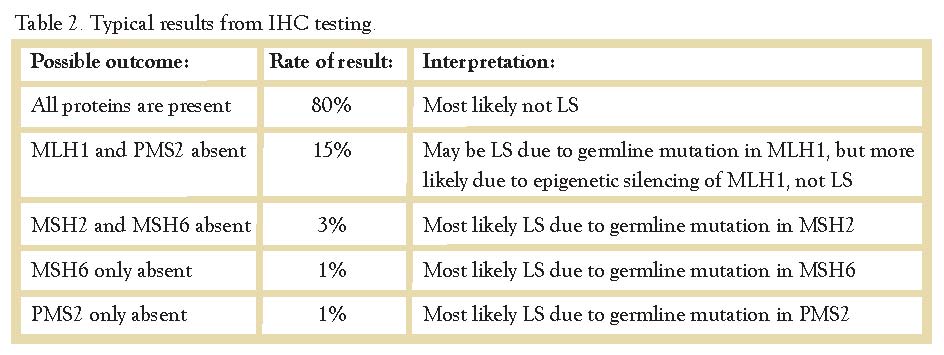
IHC is typically preferred over traditional PCR-based MSI testing because it can help direct genetic testing. Based on the results of IHC, the genetic counselor will know which gene(s) to test for. For example, with absent MSH2/MSH6, the genetic counselor will send testing for MSH2 analysis only. Testing of the other genes is not indicated because the tumor staining pattern suggests a possible mutation in MSH2 only while the other genes are likely normal. IHC is also superior to traditional PCR-based MSI testing because it is easier to perform and less expensive, and it can be performed in most laboratories, including LGH. PCR-based MSI testing is typically performed at a national reference lab or academic center.
EPIGENETIC SILENCING OF MLH1
The majority of tumors that are MSI-H or show absent staining of MLH1 and PMS2, are not related to Lynch Syndrome. Epigenetic silencing of MLH1 due to sporadic hypermethylation of the gene promoter region is responsible for as much as 80% of tumors with MSI.2 This acquired (not inherited) genetic anomaly causes the gene to be turned ‘off’ somatically resulting in loss of MLH1/PMS2 and MSI.
Therefore, when there is absent staining of MLH1/PMS2 or MSI, it is important to distinguish between Lynch Syndrome and sporadic MLH1 hypermethylation. Using PCR, hypermethylation can be detected directly, but this testing can be costly.
The V600E mutation in the BRAF gene causes acquired MLH1 hypermethylation and can be used as a surrogate marker for the presence of MLH1 hypermethylation. BRAF testing is less costly than studies of PCR-based MLH1 hypermethylation. Positive BRAF mutation testing confirms a case of sporadic CRC, but negative BRAF testing does not rule out sporadic CRC because BRAF only accounts for as much as 69% of tumors with epigenetic silencing of MLH1.2 Other causes of acquired, sporadic hypermethylation are yet unknown.
REFLEX TUMOR TESTING
In 2012, the Lancaster General Health Pathology Department implemented a reflex IHC process to aid in the identification of Lynch Syndrome patients in our community. Since the publication of recommendations on universal IHC screening by the Evaluation of Genomic Applications in Practice and Prevention (EGAPP ) Working Group,11 many institutions are establishing similar protocols. In addition to CRC, tumor testing has also been considered for Lynch Syndrome screening in endometrial and other LS tumors.
At LGH, all colorectal cancers diagnosed at age 70 or less,1 and all uterine cancers diagnosed at age 60 or less,12 are automatically tested by IHC at the time of surgical resection. If there is loss of MLH1/PMS2 on a colorectal tumor, BRAF mutation testing is undertaken as a first step in ruling out a sporadic cause. If BRAF is negative, MLH1 hypermethylation studies are then pursued. Because BRAF testing is not informative in uterine tumors,12 MLH1 hypermethylation studies are performed upfront if there is loss of MLH1/PMS2 in a uterine tumor.
Patients with abnormal staining not shown to be associated with sporadic MLH1 hypermethylation are recommended to be referred to the Cancer Risk Evaluation Program (CREP) at LGH for genetic counseling and further assessment.
GENETIC TESTING CRITERIA
If there is MSI-H or abnormal IHC (unrelated to somatic hypermethylation of MLH1), germline genetic testing is indicated to diagnose Lynch Syndrome. Of the 19 patients with abnormal IHC (Table 3), three (16%) were found to carry a mutation in an MMR gene, two carried a mutation in MLH1, and one patient carried a mutation in MSH2 (refer to patient’s pedigree in Figure 2 on the right).
About 90% of CRC patients with Lynch Syndrome will have MSI-H/abnormal IHC,2,11 meaning there is approximately a 10% false negative rate with MSI/IHC tumor testing when screening for Lynch Syndrome. Therefore, even when tumor testing is negative/normal, genetic testing may still be recommended for patients who meet all of the following ‘Amsterdam’ genetic testing criteria:
- There must be at least 3 relatives with CRC/Lynch cancers;
- One person must be a first-degree relative of the other two;
- At least two successive generations must be affected;
- At least one of the individuals must have been diagnosed prior to age 50.
Referral to a genetic counselor (GC) or other qualified genetics provider is essential for the testing process. A GC will provide thorough and non-directive education/counseling to assist the patient in making an informed decision about whether or not to have genetic testing. Complicated cases may require additional considerations beyond Lynch Syndrome, and the GC is equipped to identify other potential genetic risk factors. Patients will also benefit from the knowledge and expertise of the GC in ordering the most appropriate testing and acquiring insurance approval.
REFERENCES
1. NCCN Guidelines for Genetic/Familial High Risk Assessment: Colorectal. Version 2.2014. NCCN.org
2. de la Chapelle A and Hampel H. Clinical relevance of Microsatellite instability in colorectal cancer. JCO. 2010 Jul;28(20):3380–3387.
3. Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, et al. A national Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. CancerRes. 1998 Nov;58:5248–57.
4. Umar A, Boland CR, Terdiman JP, Synga LS, de la Chapelle A, Ruschoff J, et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J NatlCancerInst. 2004 Feb;96:261–8.
5. Heinimann K. Toward a molecular classification of colorectal cancer: the role of microsatellite instability status. Front. Onc. 2013 Oct;3:article 272.
6. Sargent DJ, Marsoni S, Monges G, Thibodeau SN, Labianca R, Hamilton SR, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. JCO. 2010 Jul;28(20):3219-3226.
7. Shia J. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part I. The utility of immunohistochemistry. J Mol Diagn. 2008 Jul;10(4):293-300.
8. Lindor NM, Burgart LJ, Leontovich O, Goldberg RM, Cunningham JM, Sargent DJ, et al. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. JCO 2002 Feb;20(4):1043-1048.
9. Moreira L, Balaguer F, Lindor N, de la Chapelle A, Hampel H, Aaltonen LA, et al. Identification of Lynch Syndrome among patients with colorectal cancer. JAMA. 2012 Oct;308(15):1555-1565.
10. Hampel H, Frankel W, Martin E, Arnold M, Khanduja K, Kuebler P et al. Feasibility of screening of Lynch Syndrome among patients with colon cancer. JCO. 2008 Dec;26(35):5783-5788.
11. Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet Med. 2009 Jan;11(1):35-41.
12. Buchanan DD, Tan YY, Walsh MD, Clendenning M, Metcalf AM, Ferguson K, et al. Tumor mismatch repair immunohistochemistry and DNA MLH1 methylation testing of patients with endometrial cancer diagnosed at age younger than 60 years optimizes triage for population-level gremlin mismatch repair gene mutation testing. JCO. 2014 Jan;32(2): 90-100.