 Click to Print Adobe PDF
Click to Print Adobe PDF
Winter 2014 - Vol.9, No.4
|
Laparoscopic Vertical Sleeve Gastrectomy for Obesity
Joseph McPhee, M.D., F.A.C.S.; James Ku, M.D., F.A.C.S., F.A.S.M.B.S.; Lawrence Wieger, D.O.; Steven Johnson, B.S.
|
  |
INTRODUCTION
The obesity epidemic continues to be a significant health burden for the U.S. population. 68% of adults are overweight or obese, according to the most recent data from the National Health and Nutrition Examination Survey (NHANES). Within that cohort, 36% exhibited class I or greater obesity (BMI > 30 kg/m2) and 6.3% were class III or morbidly obese (BMI>40 kg/m2). Over the last 10 years the number of morbidly obese adults has doubled and it is predicted that 90% of the US population will be overweight or obese by the year 2030.1,2 With the concurrent increase in diabetes, hypertension, dyslipidemia, cancer, heart disease, and sleep disorders, by the year 2030 the medical cost for obesity in the US could reach $861 billion dollars.3
According to the 1991 NIH consensus, bariatric surgery is the most reliable and durable treatment for the morbidly obese (BMI >40),4 yet only about 179,000 weight loss procedures were performed in the U.S. in 2013. That experience means that only 1% of patients who could benefit from metabolic surgery actually received it.5
EVOLUTION OF SURGERY FOR OBESITY
A variety of surgical procedures have been introduced over the past 50 years in an effort to find the safest and most effective approach to fighting the already losing battle against obesity. The sleeve gastrectomy is currently the newest and most popular procedure, but it would be worth looking back at the evolution of weight loss surgery in order to understand how we have gotten to today’s position.
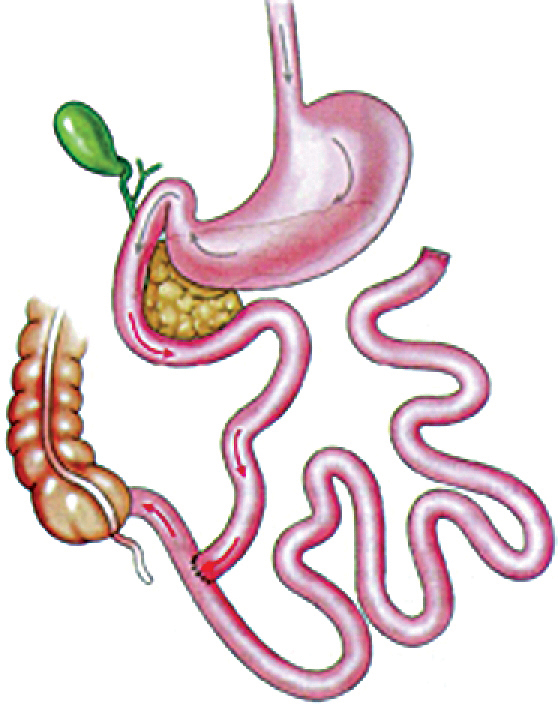
a. The first procedure developed to specifically induce weight loss for the morbidly obese was the jejuno-ileal bypass. Initially performed in the 1950’s, it was intended to produce a state of malabsorption by excluding most of the small intestine, leaving only about 50 cm for absorption (Figure 1). Although it was effective in producing weight loss, it caused disastrous complications. Over 30,000 procedures were performed, but eventually most needed to be reversed due to the side effect of severe vitamin and mineral deficiencies, protein malnutrition, and liver failure.
Fig.1
b. In the 1960’s the Roux-en-Y gastric bypass (RYGB) was developed by Drs. Mason and Ito after observing the weight loss that followed partial gastrectomy for peptic ulcers. Fifty years later it remains one of the most commonly performed weight loss procedures, and though specific surgical details have evolved, its basic concept is unchanged. A small stomach pouch, estimated at 30 cc, is constructed, and a portion of intestine is “bypassed,” most often in a Roux-en-Y fashion (Figure 2).
Fig. 2.
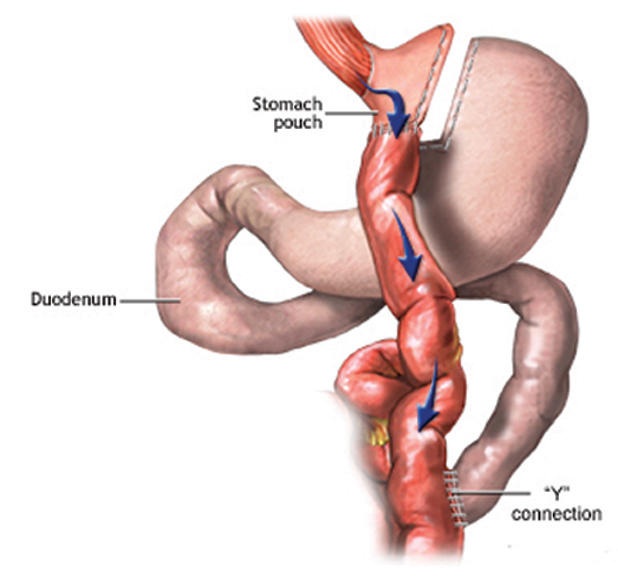
Due to the lessons learned from complications of the jejuno-ilieal bypass, the length of the bypass has been reduced, usually to between 75 and 200 cm. This method has vastly decreased the incidence of severe forms of protein and macronutrient malabsorption. Today, the risks and complications of laparoscopic gastric bypass procedures are drastically reduced from the open procedures of the past. Mortality remains low, ranging from 0.3% to 1.0%. The major morbidities are anastomotic leak (2%), hemorrhage (1.9%), and bowel obstructions (1.7%).6
c. The vertical banded gastroplasty (VBG) was developed by Dr. Edward Mason in the early 70’s as an alternative to the RYGB. Instead of focusing on malabsorption with its inherent risk of vitamin and mineral deficiencies, the VBG works by creating a restrictive state that causes early satiety. It involves creating a tubular pouch on the lesser curvature with an undivided vertical staple line, and placing a polypropylene mesh band at the pouch’s outlet (Figure 3). It became a very popular operation because it eliminated micronutrient deficiencies and had a lower risk of postoperative complications in the absence of an intestinal anastomosis. However, after thousands of operations had been performed, it was eventually seen that its benefits weren’t very durable. There was often prominent weight regain, formation of fistulas through the undivided staple line, and exacerbation of GERD. Although there are still some surgeons performing the VBG, it has slowly been replaced over time with another purely restrictive operation, the adjustable gastric band (Figure 4).
Fig. 3.
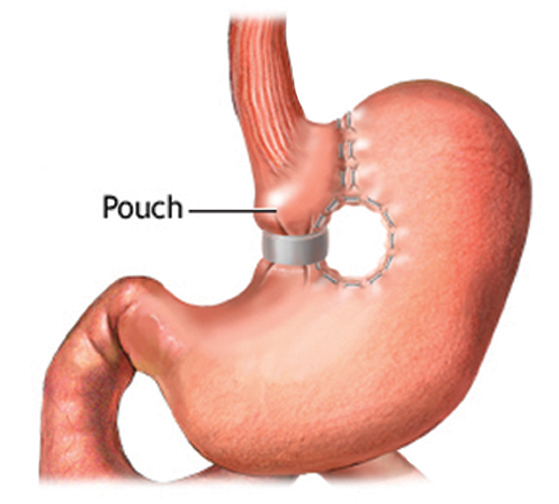
Fig. 4.
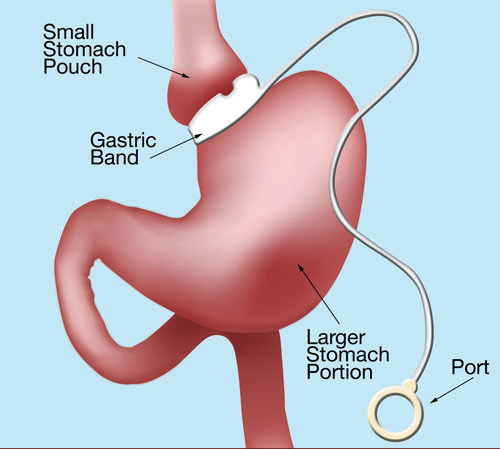
d. Although a number of non-adjustable bands were introduced starting in 1978, the first adjustable gastric band (AGB) was placed in 1986 by Dr. Lubomyr Kuzmak, a private surgeon working in New Jersey. It was a silicone-lined band with an inflatable balloon attached to a port placed underneath the abdominal wall (Figure 4). As with the VBG it is a purely restrictive operation. The underlying concept was that the band could be filled gradually over time, to the individual patient’s feeling of early satiety. Initial results from Australia and Europe were extremely positive.7,8 The AGB became a weight loss tool in the US starting in 2001 when the FDA approved the Lap-Band system. For years it was the main alternative to the RYGB and became a popular option for those who wanted to avoid the more complex operation. Although the initial mortality and early morbidity data were positive, long term results have been disappointing in terms of sustained weight loss. Over time the major reoperation rate approaches 22% of patients and there is nearly a 40% failure rate at 5 years.9,10
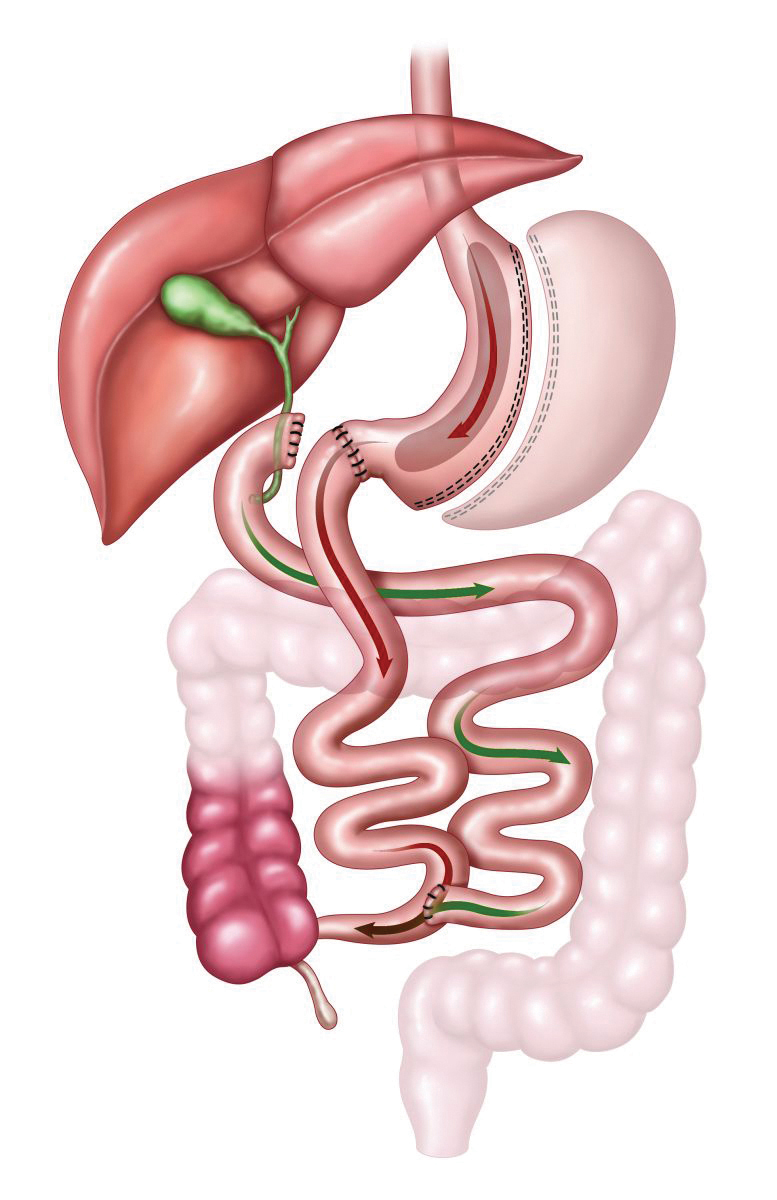
e. About the same time as the development of the adjustable gastric band, Scopinaro was developing an alternative to the RYGB by designing another operation that causes malabsorption. Unlike the RYGB, the biliopancreatic diversion (BPD) involves removing 70% of the stomach in a horizontal fashion, mainly to decrease acid production; the remaining portion is far larger than the gastric pouch after gastric bypass. The bowel is divided at the duodenum and a very long biliopancreatic limb is formed, thus leaving only 50-100 cm of bowel for food absorption (Figure 5). As can be expected, the weight loss achieved is excellent, although given the length of bypass, problems with nutritional, vitamin and mineral deficiencies can be significant.
Fig. 5.
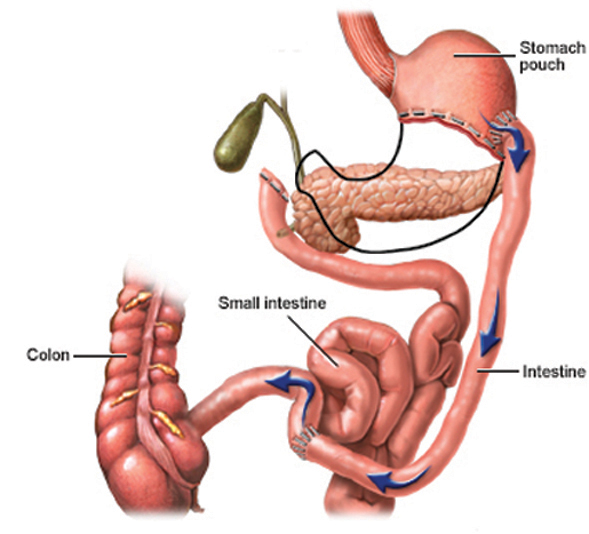
f. In 1986 Dr. Doug Hess modified the BPD to decrease problems with protein deficiencies, ulcers, and the dumping syndrome. The Duodenal Switch (DS) (Figure 6) works by using both restriction and malabsorption, as does the RYGB. A large portion of the stomach is removed, but the remainder is formed into a tube that preserves the pylorus, rather than the large pouch one sees with the BPD (Figure 5). This creates a feeling of early satiety similar to that felt with a bypass. With the knowledge that the duodenum is more tolerant to acid than the small bowel, the DS involves an anastomosis between the duodenum and small bowel. The duodenum is cut 2-4 cm from the pylorus and the intestine is sewn to the end of the duodenum that remains in continuity with the stomach. The remaining bypass is similar to that of the BPD (Figure 6). This procedure has some of the highest rates of weight loss but also arguably has the highest rate of nutritional complications compared to the RYGB and the purely restrictive operations.
Fig. 6.
g. Up to this time all these procedures had been performed through a standard laparotomy incision. It wasn’t until 1993 that the first laparoscopic RYGB was performed by Drs. Alan Wittgrove and Wesley Clark.11 That same year, a Belgian general surgeon, Dr. Mitiku Belachew performed the first laparoscopic Adjustable Gastric Band (AGB).12 In 1999, Dr. Michel Gagner performed the first laparoscopic Duodenal Switch, showing that even the most complex weight loss operation could be performed safely with laparoscopy.13
LAPAROSCOPIC SLEEVE GASTRECTOMY
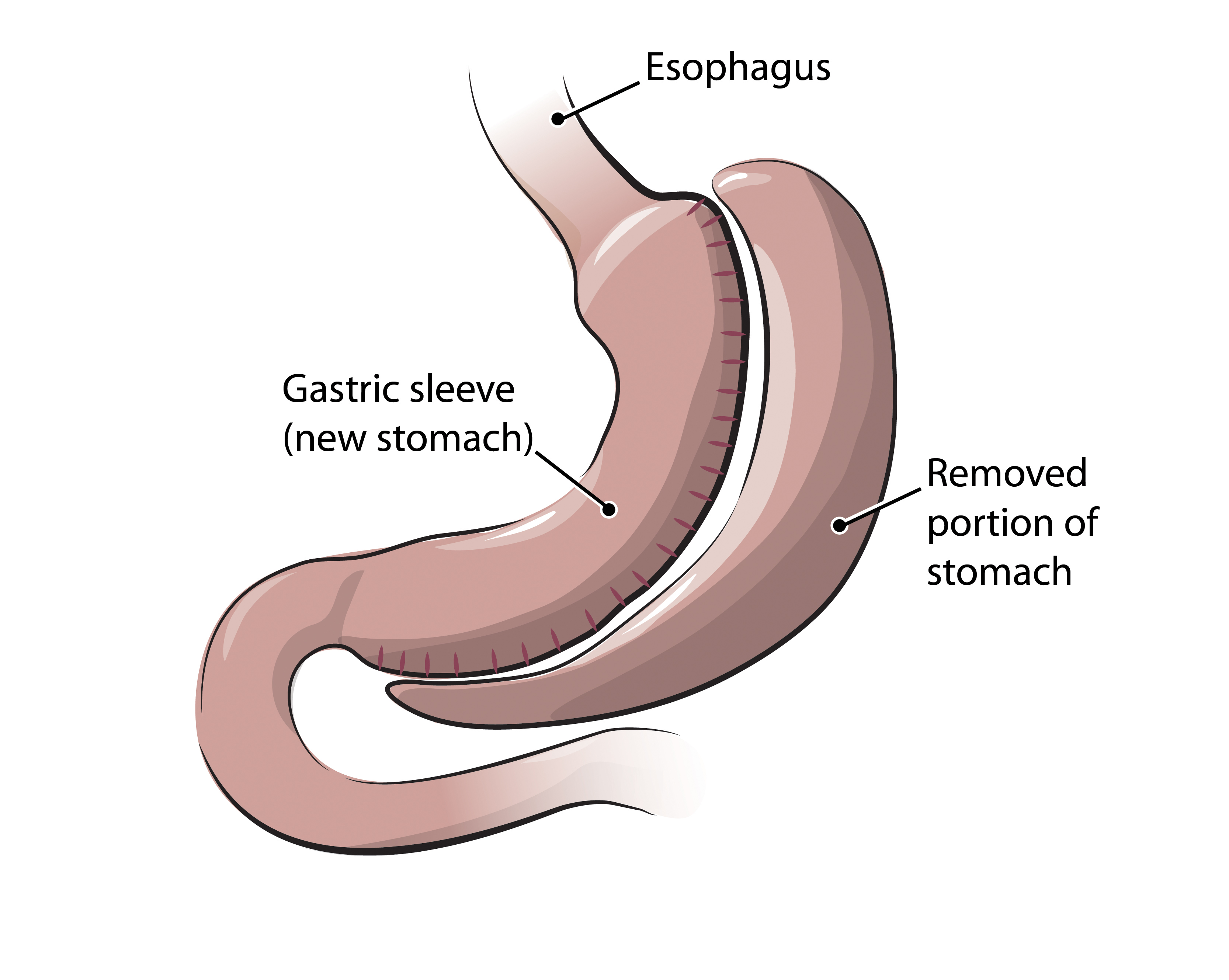
It is notable that laparoscopic sleeve gastrectomy was first mentioned at that time, almost 15 years ago. It was already well known that the more severely obese patients had a higher risk of complications. Dr. Gagner was aware of these potential complications and while performing one particularly difficult Duodenal Switch he decided to stop the case after completing only the lesser curvature based gastric tube, or “sleeve” as it came to be known (Fig. 7). The patient lost more than 100 pounds over the next year, and Dr Gagner saw an opportunity to reduce the complications in the super-super obese (BMI >60 kg/m2) by doing the DS in two parts. He first performed the relatively simple sleeve portion of the operation, and after weight loss occurred he would re-operate to complete the intestinal portion. He then looked at his RYGB patients that were super-super obese and applied the same principle.14 Over time, he realized that many patients were losing a significant amount of excess body weight with the sleeve gastrectomy alone, and it was not necessary to bring them back for the second operative procedure.
Fig. 7.
Two years later Dr. Aniceto Baltasar published the first series of laparoscopic sleeve gastrectomies as a primary procedure for weight loss.15 He performed the operation on a variety of patients including those who were super-obese, had severe medical disease, had a lower BMI, or needed a conversion procedure. His results were impressive, with only one death in a patient with a BMI of 74, for a mortality rate of 3.2%. The percentage of BMI loss in his patients approached that obtained with RYGB, and he concluded, “The SG appears to be an improvement over the prior gastroplasty procedures, which have problems related to the placement of staples, silastic rings, circumferential mesh, and gastro-gastric fistula. No foreign body is used unlike the gastric banding.”15
Surgeons began to take notice of this improved, purely restrictive operation. At the first International Consensus Summit for Sleeve Gastrectomy in 2007, when SG represented only 2% of the metabolic operations being performed in the U.S., they began looking at long-term weight loss, which had been the downfall of other restrictive procedures. They found that at 3 years the percentage of excess body weight loss (%EBWL) was 56%, which was very similar to that obtained with the bypass procedure, but without its problems of malabsorption, thus verifying Dr. Baltasar’s findings of two years earlier.16
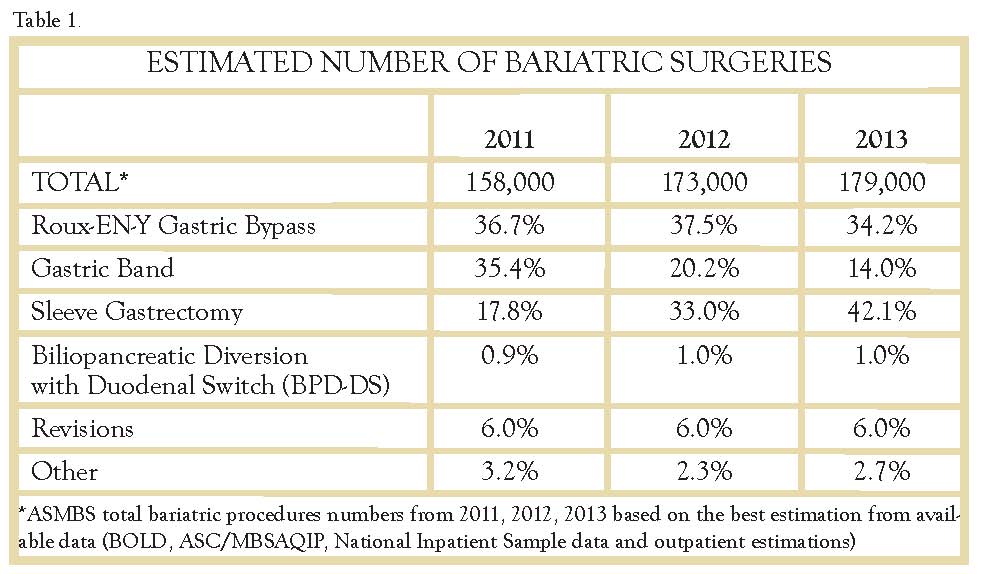
Further studies began to examine the safety, mechanism, and efficacy of this new approach to surgical weight loss,17-20 culminating in a consensus statement in 2011 by an international sleeve gastrectomy expert panel.21 Substantial 5 year data were available by this time, with a combined experience of over 12,000 cases. It was the first combined consensus which focused exclusively on guiding clinical practice, surgical technique, and future research regarding laparoscopic sleeve gastrectomy. At this time acceptance of the sleeve procedure by insurance companies was improving, but it was still a problem. Since then the popularity of the operation has skyrocketed. Its safety, simplicity, and long term weight loss and resolution of comorbidities have made the SG the most popular operation performed in 2013 (Table 1). The numbers of sleeve gastrectomies performed in our practice show a very similar trend with 16 cases performed in FY2012, 101 cases in FY2013, and 204 cases in FY 2014.
The operation is done by removing two thirds of the stomach, mostly the gastric fundus, and making a vertical sleeve based on the lesser curvature, thus causing early satiety. The diameter of the sleeve varies between 32F-40F (roughly 1-1.3 cm) and its distance from the pylorus varies slightly from 2 cm to 6 cm (Figure 7). In retrospect it was found that the operation works not only by causing early satiety, but because the gastric fundus produces 2/3 – 3/4 of Ghrelin, a 28 amino acid gut peptide that is pivotal in the stimulation of appetite. As an endogenous hormone, Grehlin binds to the receptor for the growth hormone secretagogue and stimulates the hypothalamus to release growth hormone, thereby playing an important role in weight regulation. Removal of the majority of the greater curvature of the stomach removes cells that produce Ghrelin, thus decreasing appetite stimulation. The combined effect of early satiety and decreased hunger drive is the reason such significant weight loss is achieved with a purely restrictive procedure. Because there is no manipulation of the intestine there are no side effects of malabsorption. An additional positive feature of the sleeve gastrectomy is that direct access to the entire upper GI tract is maintained and there is no contraindication to post-operative use of medications. Patients lose an average of 60% of their excess body weight at one year, accompanied by the expected improvement (or resolution) of obesity-related diseases.22,23 The operation is totally laparoscopic, takes an average of 45 minutes, and patients tend to go home the day after surgery. As with other laparoscopic operations the vertical sleeve gastrectomy is a very safe operation, with a postoperative 30-day mortality rate of 0.19%. Major complications include staple line leak (2.2%), bleeding (1.2%), and stricture (0.63%).24
CONCLUSION
Weight loss surgery has been a very dynamic field over the last 60 years. Many lessons have been learned, with peaks and valleys of successes and failures. With the obesity epidemic continuing to rise, and with no end in sight, we must continue to look for the safest and most effective weight loss procedures. Laparoscopy was a giant step in that direction and the sleeve gastrectomy appears to be a reliable and durable option in the surgical arsenal for the ongoing battle against obesity.
REFERENCES
1. Flegal KM, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in the distribution of body mass index among US adults 1999-2010. JAMA 2012;307 (5):491-497.
2. Ogden CL, Caroll MD, Curtin LR et al. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295 (13): 1549-1555.
3. Wang Y, Beydoun MA, Liang L, et al. Will all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity. 2008;16(10):2323-2330
4. National Institutes of Health Consensus Development Panel. Gastrointestinal surgery for severe obesity. Ann Int Med 1991;115:956-961
5. New Procedure Estimates for Bariatric Surgery: What the Numbers Reveal. Connect: the official news magazine of the AMBS (http://connect.asmbs.org/may-2014-bariatric-surgery-growth-html)
6. Pondos YD, Jimenez JC, Wilson SE et al. Complications after laparoscopic gastric bypass: a review of 3464 cases. Arch Surg 138 (9):957-961
7. Suter M, Giusti V, Heriaef E et al. Early results of Laparoscopic gastric banding compared with open vertical banded gastroplasty, Obes Surg 1999;9:374-380
8. Fielding GA, Rhodes M, Nathanson LK. Laparoscopic gastric banding for morbid obesity. Surgical outcome in 335 cases. Surg Endosc 1999;13: 550-554
9. Boza C, Gamboa C, Oerez P. Laparoscopic adjustable gastric banding (LAGB): surgical results and 5 year follow-up, Surg Endosc 2011;25:292-297
10. Suter M, Calmes JM, Paroz A. A 10-year Experience with Laparoscopic Gastric Banding for Morbid Obesity: High Long-Term Complication and Failure Rates. Obes Surg 2006;16:829-835
11. Wittgrove A, Clark W, Temblay L. Laparoscopic Gastric Bypass, Roux-en-Y: Preliminary Report of Five Cases. Obes Surg 1994;4:353-357
12. Belachew M, Legrand M, Vincent V, et al. Laparoscopic Adjustable Gastric Banding. World J Surg 1998;22:955-963
13. Ren C, Patterson E, Gagner M. Early Results of Laparoscopic Biliopancreatic Diversion with Duodenal Switch: A case Series of 40 Consecutive Patients. Obes Surg 2000;10:514-523
14. Regan JP, Inabnet WB, Gagner W. Early Experience with Two-Stage Laparoscopic Roux-en-Y Gastric Bypass as an alternative in the Super-Super Obese Patient. Obes Surg 2003;13:861-864
15.Baltasar A, Serra C, Perez N, et al. Laparoscopic Sleeve Gastrectomy: A Multi-purpose Bariatric Operation. Obes Surg 2005; 15:1124-1128
16. Deitel M, Crosby, R, Ganger, M. The First International Consensus Summit for Sleeve Gastrectomy (SG), New York City, October 25-27, 2007. Obes Surg 2008; 18: 487-496
17. Melissas J, Daskalakis M, Koukouri S, et al. Sleeve Gastrectomy- A “Food Limiting” Operation. Obes Surg 2008;18:1251-1256
18. Akary E, Duffy A, Bell R. Deciphering the Sleeve: Technique, Indications, Efficacy, and Safety of Sleeve Gastrectomy. Obes Surg 2008;18:1323-1329
19. Basso N, Casella G, Rizzello M. Laparoscopic sleeve gastrectomy as first stage or definitive intent in 300 consecutive cases. Surg Endosc 2011; 25: 444-449.
20. Lee CM, Cirangle PT, Jossart GH at al. Vertical gastrectomy for morbid obesity in 216 patients: report of two-year results. Surg Endosc 2007; 21 (10): 1810-1816
21. Rosenthal R. International Sleeve Gastrectomy Expert Panel Consensus Statement: best practice guidlines based on experience of > 12,000 cases. SOARD. 2012; 8: 8-19
22. Himpens J, Dapri G, Cadiere GB et al. A prospective randomized study between laparoscopic gastric banding and laparoscopic isolated sleeve gastrectomy: results after 1 and 3 years. Obes Surg 2006; 16 (11): 1450-1456
23. Basso N, Casella G, Rizzello M, et al. Laparoscopic sleeve gastrectomy as first stage or definitive intent in 300 consecutive cases. Surg Endosc 2011; 25: 444-449
24. Updated Position Statement on Sleeve Gastrectomy as a Bariatric Procedure. Clinical Issues Committee of the American Society for Metabolic and Bariatric Surgery. SOARD 2010; 6: 1-5