
Fall 2014 - Vol. 9, No. 3
The Micra Transcatheter Pacing Study:
The Making of a Revolution in Pacemaking
Matthew A. Bernabei, M.D.
Lancaster Heart and Vascular Institute
BACKGROUND
There is a major paradigm shift underway in the world of implantable devices for management of cardiac rhythms. Technological advances in miniaturization and fusion of device components have led to the development of a device with a completely novel platform and procedure. This device promises to make implantation even less invasive, while at the same time obviating many of the complications inherent in the ‘old’ technology and procedure. We have begun enrolling patients in a clinical trial of one such device at our institution. It seeks to assess the safety and efficacy of the world’s smallest, “leadless” pacemaker in patients with symptomatic bradycardia, where single-chamber (ventricular) pacing support is needed. Though this initial study of the new technology will apply only to a rather small, focused subset of patients (both during, and likely after the trial), the technology is widely expected to expand to all implantable cardiac devices in the near future.
For more than 50 years, the procedure for implanting a traditional pacemaker for bradycardia has been relatively unchanged. While the ‘software’ inherent in these devices has gradually evolved (marked mostly by improved pacing algorithms), the basic design of the ‘hardware’ has remained constant in its use of a lead/wire and a generator/battery. The constraints imposed by this design have prevented any significant changes or advances in the overall procedure, which still requires an incision below the clavicle of approximately 3-inches, to create a pocket for the generator. The axillary/subclavian vein near this incision is then accessed, and a lead is advanced through the venous system to the heart. After stable contact with the ventricular endocardium is assured, the other (pectoral) end of the lead is secured to the chest wall, and the end of the lead is attached to the generator with a screw. The generator is then implanted in the pocket, and the wound is closed (Fig. 1).
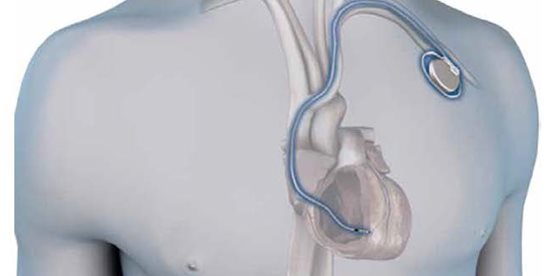
Fig. 1. The traditional, transvenous pacemaker system involving a generator implanted medial to the left delto-pectoral groove and inferior to the clavicle, and a lead implanted through the axillary/subclavian vein and extending through the superior vena cava into the heart.) (Reprinted with permission, Medtronic Inc.)
This procedure has proven quite robust through the years, with limited risk and a low frequency of acute complications like pneumothorax, perforation or hematoma. Nonetheless, as time passes the implanted generator and leads can cause many well-known complications such as: erosion of the generator through the skin; infections of the wound and device; malfunction of the lead due to fracture or breach of the insulation; issues related to vascular access (such as use of veins needed for dialysis) or vein stenosis caused by the lead itself and therefore complicating “lead management” should a new lead be required.
THE LEADLESS PACEMAKER
With all of this in mind, device makers have endeavored for years to engineer a way around these technological limitations and their associated complications. In the clinical trial in which we are taking part (Micra Transcatheter Pacing Study), the sponsor (Medtronic, Inc.) has devised a way not only to shrink the generator by more than 90% (while at the same time improving battery life), but also to meld the tip of the lead directly to this smaller generator (Fig. 2), thus yielding a fully integrated, self-contained, ‘single-unit’ pacemaker that has no lead. Because of its diminutive size and lack of wires, it can be delivered directly to the heart using a catheter-based delivery system from the groin. In addition to avoiding the aforementioned complications, this design leads to another notable benefit: the absence of any outward sign—such as a scar or a bulging pectoral device—that indicates the patient even has a pacemaker.

Fig. 2. To the left is a traditional pacemaker with an attached lead. To the right is the entire Micra device, a “leadless” pacemaker marked by a significantly smaller generator, with the lead tip attached directly to the bottom of the device, in between the fixation tines.
First some basic information: the research device (Micra) is 2.5cm long (1 inch); 0.75cc in volume (compared with 10 cc for a traditional pacemaker); the approximate width of a pencil (in this case 20FR, or about 7mm); and again, has no leads or wires. It has been described as the size of a vitamin. It adheres to the myocardium using a novel fixation mechanism that is comprised of four Nitinol (Nickel-Titanium Alloy) tines that are packaged and presented in a retracted state for introduction into the heart, but resume their natural, ‘grappling’ state when deployed during delivery of the device, which allows them to actively grab the underlying myocardial trabeculae (Fig. 3).
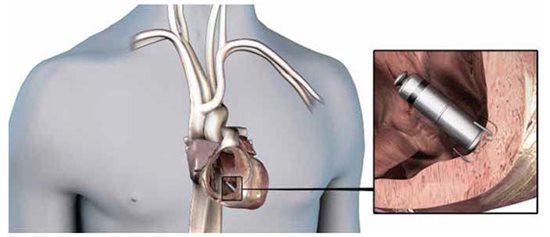
Fig. 3. The Micra device fully deployed in the right ventricular apex and after the delivery catheter has been removed. Note the Nitinol tines grappling the endocardium, which serve as the fixation mechanism, but also as an upward, fulcrum-like spring that pulls the lead tip down into the muscle, which contributes to greater battery longevity. (Reprinted with permission, Medtronic Inc.)
The “lead tip” (if we can still call it that) or cathode is on the bottom of the device/generator in between these tines. When the tines are deployed, they serve as a type of fulcrum-like ‘spring’ which actively pulls the lead tip down into better contact with the endocardium. This improves pacing parameters like threshold and impedance (when compared to traditional devices), reduces battery drain, and contributes to longer battery life. Further, the very absence of a lead in the first place is another notable contributor to improved battery life, since the farther energy has to travel, the more it is dissipated/wasted. In the case of leadless systems, a generator in direct contact with the muscle it is depolarizing is inherently more efficient. The Micra has an expected average battery life of about 10 years.
Because of the significantly smaller device size, as well as its leadless design, this pacemaker is delivered directly to the heart from a catheter-based approach from the femoral vein (an approach already familiar to electrophysiologists, as this is how ablation is performed.) The device itself is packaged inside the tip of the catheter, allowing the tines to be retracted and covered on entry into the heart. As the system is advanced to the right atrium, it is then curved so that the catheter tip holding the Micra prolapses across the tricuspid valve toward the right ventricular apex (Figure 4). Once good contact is confirmed with fluoroscopy, the catheter is slowly withdrawn while the Micra is carefully advanced, which uncovers the tines and allows them to engage the endocardium. At this point, the catheter is pulled back further, but is still tethered to the pacemaker by a suture, which runs through the catheter and extends out the back of the catheter handle and into the operator’s hand. By gently tugging on this suture, the anatomic stability (the tines’ “grip”) of the device is assessed over several minutes, followed by evaluation of the device’s electrical parameters. Once good contact and favorable pacing function are assured, the device is untethered from the tip of the catheter delivery system by cutting the suture in the operator’s hands. The suture is then pulled/slid out through the device’s head and the entirety of the catheter, after which the whole assembly is withdrawn from the femoral vein.
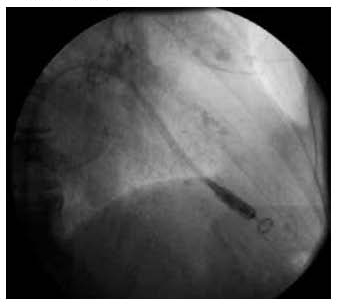
Fig. 4. The delivery catheter prolapsed across the tricuspid valve, with the pacemaker still inside its distal tip; shown here in the right anterior oblique, fluoroscopic view, and just prior to device deployment to the right ventricular apex.)
MANAGEMENT OF THE DEVICE
The methods for near-term retrieval/removal of the device, and for management at the end of battery life (which is likely to be handled differently), have appropriately generated many questions and much thought and consideration. If for some reason, the device should need to be removed, it can be recaptured using the same catheter delivery system, which after engaging the device’s head, can be snared around the neck and pulled back into the sheath. While this is relatively easy and safe in the near term (days, weeks, months, and perhaps even years), greater concern arises over the safety and efficacy of late extraction after several years– such as at the end of the device’s life. During longer periods of time the device becomes endothelialized, which makes it more adherent to, and embedded in the endocardium. This subsequently makes delayed extraction, while still achievable, much more technically challenging and inherently more risky. This consideration, combined with the already small size of the device—which is likely to shrink still further by the time the current devices reach end of life—means that the risk/benefit ratio of the future will likely favor simply adding a new device and abandoning the old one (as we often do now with old transvenous leads).
THE CURRENT TRIAL
For now (and in its first iteration), the device will be implanted as part of the study only in patients where single-chamber ventricular pacing is indicated. We implant about 30 such devices a year at our institution. The trial sponsor estimates that about 46,000 devices will be implanted in the U.S. this year that would fit into this category. The most common situation where these types of devices could be used is in patients with chronic atrial fibrillation and slow ventricular response.
THE FUTURE
Given the far-reaching implications of this disruptive technology, it is easy to imagine that such advances will soon find their way into dual-chamber pacemakers (330 implants last year at Lancaster General Hospital and approximately 247,000 in the U.S.), and biventricular devices and defibrillators (250 at LGH and 200,000 in the U.S.). There is animal work already underway in which two devices that can communicate with each other are implanted, one in the ventricle and another in the atrium, opening the door to what many expect to be the next phase—leadless dual-chamber pacing.
In a related application, subcutaneous defibrillators (S-ICD) have grown in popularity for many of the same reasons that leadless pacemakers show such promise: the ability to avoid a lead in the vasculature. The S-ICD has a generator that is placed under the skin about six inches inferior to the left axilla. An ICD lead is then tunneled under the skin from this area horizontally, along the line inferior to the left pectoral muscle, where it is then turned cranially (when it reaches the left parasternal line) and advanced toward the left sternoclavicular joint. The shock coil is left to reside completely under the skin, all the while creating a shock vector that basically encircles the heart by following the contours of the thorax. The shortcomings of this device (due to the absence of actual contact with the myocardium), however, are its inability to serve as a backup pacemaker for bradycardia, as well as the absence of anti-tachycardia pacing (ATP) (a common capability of traditional transvenous defibrillators, that can often terminate ventricular tachycardia without resorting to a shock.) Many expect future generations of S-ICDs, to not only communicate with leadless pacemakers, but to be routinely paired with them at the time of ICD implantation. Such a symbiotic union would provide the critical ‘intracardiac’ presence (through the leadless pacer), could provide both bradycardia and tachycardia pacing, and would still avoid the undesirable permanent transvenous lead. Finally, once two devices can reliably communicate with each other, we expect that a third communicating element will join the system and ultimately enable biventricular pacing. Given the already small sizes achieved with these devices, it does not seem too far-fetched to think that these could also provide cardiac resynchronization by being introduced through the coronary sinus and ‘wedged’ in a favorable, epicardial venous branch of the left ventricle. Alternatively they might even be placed endocardially in the left ventricle. Already one company (EBR Systems) has been investigating the potential for Wireless Cardiac Stimulation of the left ventricle with one such device (WiCS-LV).
FINAL THOUGHTS
It appears that the makings of a true revolution in device therapy may well be underway. While there are certain to be ‘bumps’ throughout the process—as seem inherent in any innovative endeavor or technological advance—it does appear that leadless pacemakers are well positioned to replace traditional transvenous devices in the not-too-distant future. We successfully implanted our first Micra on August 4th of this year (Figures 5 and 6). Everything went very well, and we look forward to gaining more experience with this device throughout the trial. Moreover, this trial seems a good opportunity to help keep the Lancaster Heart and Vascular Institute on the forefront and ‘cutting-edge’ of cardiac rhythm management, while allowing us to offer our patients the most promising new technology.


Fig. 5 and 6. Post-procedural right anterior (left) and left anterior oblique (right) chest X-rays, showing the final position (arrows) of the device in the right ventricular apex, in our first case at Lancaster General Hospital.