
Spring 2014 - Vol. 9, No. 1
Esophageal Adenocarcinoma and Barrett's Esophagus: Current Management: Role of Radiofrequency Ablation
Ketan Kulkarni, M.D.
Regional Gastroenterology Associates of Lancaster
INTRODUCTION
The prognosis of patients with esophageal cancer continues to remain poor. Most esophageal cancers are either adenocarcinoma or squamous cell carcinoma, which are differentiated by their pathobiology and risk factors. While the incidence of esophageal squamous cell carcinoma has declined in the United States, the incidence of esophageal adenocarcinoma has risen dramatically over the past several decades.1-4 Risk factors for the development of esophageal adenocarcinoma include smoking, obesity, chronic GERD, the presence of a hiatal hernia, age greater than 50, male gender, Caucasian race, and Barrett’s esophagus (BE). Although the reported rise in the incidence of esophageal adenocarcinoma in the US has certainly been affected by improved detection and the increasing rate of obesity, there are likely important environmental factors that have not yet been identified. Since most esophageal adenocarcinomas develop in an area of Barrett’s esophagus, it is the most important of the known risk factors. The other risk factors are influential mainly because they are associated with an increased incidence of BE.
BARRETT’S ESOPHAGUS
BE is a premalignant condition in which columnar epithelium replaces the stratified squamous mucosa that normally lines the esophagus.5,8 The onset of malignancy within Barrett’s esophagus is characterized by a progression from non-dysplastic BE through increasing degrees of dysplasia and ultimately to adenocarcinoma.8-10 Patients with BE have a 30 to 40 fold increased risk of developing adenocarcinoma, which results in an annual incidence of adenocarcinoma in patients with BE of approximately 0.5%.1,2,8,11
Barrett’s esophagus itself does not result in any symptoms and in many individuals is discovered incidentally during an upper endoscopy. BE is typically found in middle-aged and older individuals and is more prevalent among males. Chronic gastroesophageal reflux disease (GERD) results in inflammation which in turn can lead to specialized intestinal metaplasia that is characteristic of Barrett’s esophagus. However, BE can also develop in individuals with little or no GERD, as more than 40% of patients with Barrett’s esophagus do not have significant symptoms of heartburn.12 Research into the genetics and molecular pathogenesis of BE is ongoing to help understand why only some patients will develop Barrett’s esophagus and why only a small portion of these individuals will progress to cancer.
MANAGEMENT OF PATIENTS WITH BARRETT'S ESOPHAGUS
Since Barrett’s esophagus is a premalignant condition and the outcome of patients with esophageal adenocarcinoma is strongly associated with their stage at the time of diagnosis, it is reasonable to hypothesize that screening for BE or surveillance of patients with known BE would help improve clinical outcome.3,13 As many as 95% of patients who undergo resection of esophageal adenocarcinoma do not have their underlying BE discovered until their diagnosis of cancer, which suggests that the opportunity to detect disease at an earlier stage is being missed in a significant proportion of individuals with adenocarcinoma.14 The American College of Gastroenterology recommends regular surveillance of patients with Barrett’s esophagus, with varying endoscopic intervals depending on the severity of dysplasia.15 However, it is important to note that endoscopic surveillance for Barrett’s esophagus has never been proven by a randomized controlled trial to improve survival.15,16 Furthermore, studies have demonstrated that surveillance is likely not cost-effective. The reason for this counterintuitive finding is that the absolute risk for patients with BE to develop esophageal cancer is very low; the overwhelming majority of patients with Barrett’s esophagus will die with their disease rather than because of it.
Similarly, the effectiveness of screening for the presence of Barrett’s esophagus is unclear, although prior studies have suggested that screening of patients at high risk for BE may be cost effective.17 As a result, current guidelines do recommend screening in patients with multiple risk factors for esophageal adenocarcinoma, which, as noted earlier, include chronic GERD, the presence of a hiatal hernia, age greater than 50, male gender, Caucasian race and obesity.18
There are a variety of management options for patients with Barrett’s esophagus with high grade dysplasia. The actual risk of progressing to esophageal cancer in patients with high grade dysplasia is likely from 4% per year up to 50% over 5 years19,20 so management of these patients is particularly important. Treatment options range from an intensive surveillance program with frequent upper endoscopies, to endoscopic therapy, to esophagectomy.
ENDOSCOPIC APPROACHES TO BARRETT’S ESOPHAGUS
Since esophagectomy is associated with significant morbidity, there has been great interest in the development of minimally invasive, endoscopic approaches that can treat Barrett’s esophagus without a major surgical procedure. For years researchers have been attempting to design a method that would ablate Barrett’s esophagus and subsequently allow the growth of new squamous epithelium, free of BE, as the mucosa heals. A variety of methods have been investigated, including photodynamic therapy, cryotherapy, argon plasma coagulation, and multipolar electrocoagulation. However, none of these earlier methods was able to provide effective and durable results with minimal side effects using a technique that was easy to perform and very reproducible.
Radiofrequency Ablation for Barrett’s Esophagus
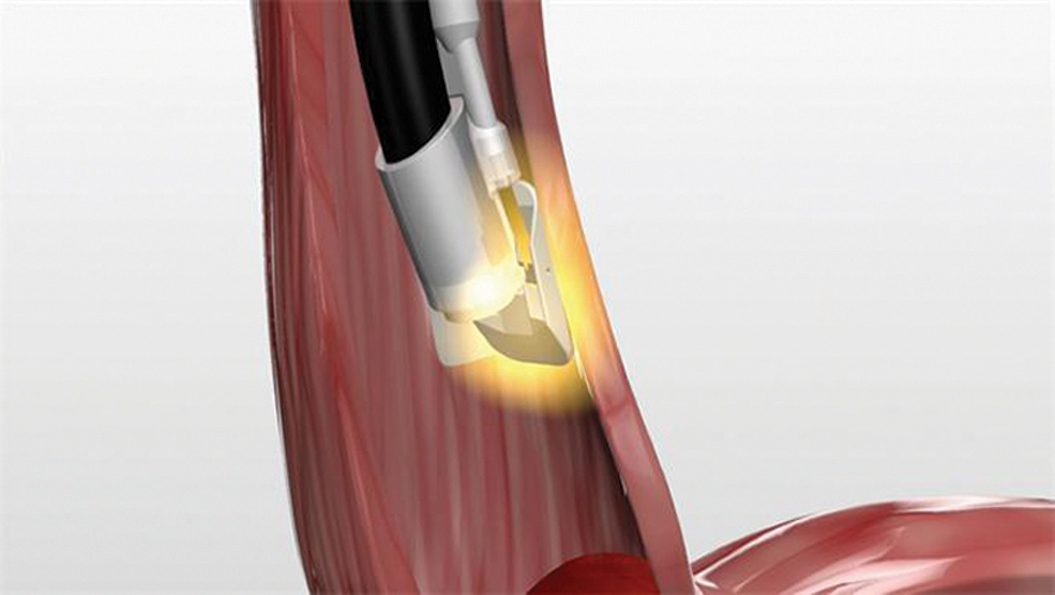
Radiofrequency ablation is a newer ablative method that has shown tremendous promise in the treatment of individuals with Barrett’s esophagus. Radiofrequency ablation (RFA), which was recently introduced at Lancaster General Hospital, is performed using the HALO system. After an RFA catheter is placed into the esophagus and guided by upper endoscopy (Fig. 1), electric current in the form of radiofrequency energy is applied to the Barrett’s esophagus.21 The catheter has an inflatable balloon to allow circumferential treatment of the esophagus (Fig. 2). A separate catheter can be utilized for focal or spot treatment of isolated tongues or for secondary “touch ups” after initial circumferential therapy (Fig. 3). After initial ablative therapy a repeat endoscopy is performed 12 weeks later to re-assess the esophagus and to perform further ablation of any remaining foci of Barrett’s esophagus. Typically, several sessions are needed to completely eradicate the BE.
Fig. 1. Radiofrequency ablation catheter introduced into the esophagus.
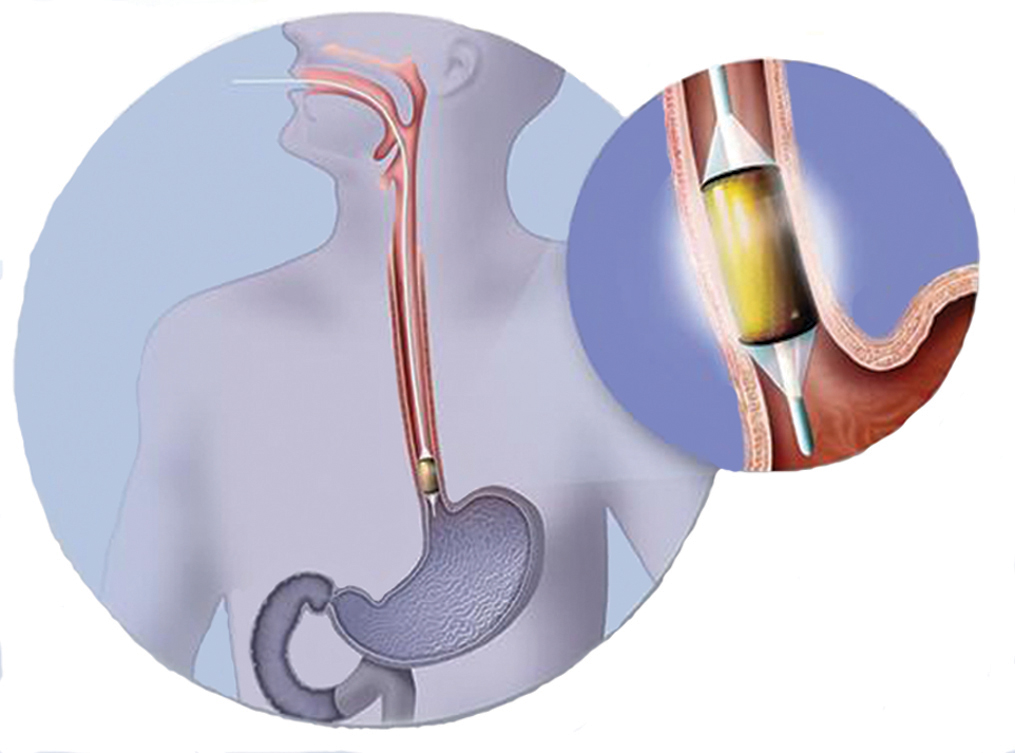
Fig. 2. Close up image of inflatable radiofrequency ablation catheter, which performs circumferential ablation of Barrett’s esophagus.

Fig. 3. Close up of the HALO 90 catheter, which allows for spot treatment of Barrett’s esophagus.
The HALO system has been designed in order to deliver the same amount of electric current with each application. Either 10 or 12 J/cm2 of radiofrequency energy is utilized depending upon the severity of dysplasia. The HALO system achieves a depth of ablation of 500 to 1000µm; this is deep enough to adequately treat Barrett’s esophagus (500 µm) but superficial enough to minimize complications such as esophageal strictures.21
A number of studies, which include randomized control trials, have demonstrated the efficacy of radiofrequency ablation and durability of the response. Shaheen and colleagues published the first major randomized, sham-controlled trial conducted at 19 centers in the United States.22 91% of patients with low grade dysplasia and 81% of those with high grade dysplasia achieved complete eradication compared with 23% and 19% in the control groups. More importantly, there was an 87% lower incidence of cancer (p<0.05) in those patients who received RFA, although the absolute number of patients that developed cancer was small. A two to three year follow up of these patients demonstrated that complete remission of dysplasia was maintained in over 90% of individuals that received intermittent touch up treatments.23 In more recently published five year data, 92% of patients with nondysplastic BE had complete eradication after 5 years. Furthermore, in patients who had a recurrence, a single session of RFA resulted in complete eradication.24
Many of the more recent studies that have evaluated RFA have investigated the combination of radiofrequency ablation and endoscopic mucosal resection. RFA has been shown to be most effective in patients with flat areas of Barrett’s esophagus, which allows the best apposition between the ablation catheter and esophageal mucosa. Endoscopic mucosal resection (EMR) is a minimally invasive endoscopic-based approach that allows gastroenterologists to remove suspicious nodular areas. With EMR, one can completely resect superficial nodular areas and provide tissue for a pathologic diagnosis. In fact patients with early esophageal intramucosal carcinoma, stage T1a that does not invade through the muscularis mucosa into the submucosa, can be completely treated by EMR without the need for esophagectomy.25 Furthermore, patients with limited areas of Barrett’s esophagus can also be treated by mucosal resection without the need for further ablation.
Nevertheless, patients typically have wider areas of Barrett’s esophagus, in which case suspicious nodules can first be resected by EMR, followed by radiofrequency ablation of the remaining Barrett’s tissue. One of the first multicenter trials investigating the combination of RFA and EMR was published by Pouw and colleagues.26 One hundred percent of patients with high grade or low grade dysplasia had complete eradication of their disease after a median follow up of 22 months. A multicenter United States registry demonstrated similar efficacy of combined endoscopic mucosal resection and RFA in the management of patients with high grade dysplasia, as 90% of patients had complete remission at 12 months.27
The American Gastroenterological Association (AGA) convened a panel of experts in 2011 to create a medical position statement on the management of Barrett’s esophagus.18 Given the significant amount of evidence demonstrating the effectiveness of RFA, the AGA does recommend that radiofrequency ablation should be a therapeutic option in patients with low grade or high grade dysplasia. They note that RFA is the most commonly used ablation procedure and there is currently inadequate literature to recommend alternative ablative techniques. Particularly for patients with high grade dysplasia, RFA should be considered a first line treatment given the morbidity associated with esophagectomy.
Controversy still exists regarding treatment of patients with BE and no dysplasia. While RFA can be considered in patients with no dysplasia who have a high risk of progression, currently we do not have appropriate criteria to identify such patients. Further, no studies have shown that endoscopic ablation is superior to surveillance in individuals with BE and no dysplasia. Other academic societies, including the American Society for Gastrointestinal Endoscopy (ASGE), echo the AGA and similarly recommend RFA for the treatment of patients with either low or high grade dysplasia.28
Despite the promising results of radiofrequency ablation, further investigation is needed to determine how durable the treatment response is. Therefore, patients that are treated with radiofrequency ablation still require endoscopic surveillance afterwards. Only time will tell if RFA will be proven to be cost effective in the long term. The major adverse effects associated with RFA include formation of esophageal strictures, transient chest pain, and gastrointestinal bleeding. Typically patients who develop a stricture can be successfully managed by endoscopic dilation. There is also concern that patients could develop buried Barrett’s, or intestinal metaplasia that is covered by neosquamous epithelium and thus is more difficult to diagnose. However, the presence of buried Barrett’s has been noted even in patients who have never been treated, so its clinical significance has not been fully elucidated.29 More recent studies have shown that the rate of buried Barrett’s after RFA is quite low, and likely lower than with any other available ablative technique.30
CONCLUSIONS
As endoscopic techniques for the treatment of Barrett’s esophagus improve and become more cost effective there will be greater rationale to treat patients in the future. Radiofrequency ablation (RFA) represents the latest technology to allow us to treat patients with Barrett’s esophagus, particularly those individuals with low grade or high grade dysplasia. The ease and reproducibility of the procedure, along with a growing body of evidence, has led to greater utilization of radiofrequency ablation for Barrett’s esophagus. More data are needed, however, to determine the long term durability of RFA. Research is ongoing to help accurately identify the patients who are at greater risk for progressing from non-dysplastic Barrett’s esophagus to cancer. Once molecular markers that predict a patient’s risk for progression are discovered, gastroenterologists will be better able to tailor treatment for each individual patient.
REFERENCES
1. Shaheen NJ. Advances in Barrett’s esophagus and esophageal adenocarcinoma. Gastroenterology 2005; 128: 1554-1566.
2. Spechler SJ. Screening for barrett’s esophagus. Rev Gastroenterol Disord 2002; 2: S25-29.
3. Lightdale CJ. Esophageal cancer. Am J Gastroenterol 1999; 94 (1): 20-29.
4. Brown LM and Devesa SS. Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clin N Am 2002; 11: 235-256.
5. Fitzgerald RC. Barrett metaplasia: reassessment of treatment and follow-up. Curr Opin Oncol 2004; 16: 372-377.
6. Shaheen N and Ransohoff DF. Gastroesophageal reflux, barrett’s esophagus, and esophageal cancer: scientific review. JAMA 2002; 287 (15): 1972-1981.
7. Shaheen NJ, Provenzale D and Sandler RS. Upper endoscopy as a screening and surveillance tool in esophageal adenocarcinoma: a review of the evidence. Am J Gastroenterol 2002; 97 (6): 1319-1327.
8. Spechler SJ. Clinical Practice. Barrett’s esophagus. N Engl J Med 2002; 346 (11): 836-842.
9. Theisen J, Nigro JJ, DeMeester TR, et al. Chronology of barrett’s metaplasia—dysplasia—carcinoma sequence. Dis Esophagus 2004; 17: 67-70.
10. Flejou JF. Barrett’s oesophagus: from metaplasia to dysplasia and cancer. Gut 2005; 54 Suppl 1: i6-12.
11. Conio M, Blanchi S, Lapertosa G, et al. Long-term endoscopic surveillance of patients with barrett’s esophagus. Incidence of dysplasia and adenocarcinoma: a prospective study. Amer J Gastroenterol 2003; 98 (9): 1931-1939.
12. Ronkainen J, Aro P, Storskrubb T, et al. Prevalence of Barrett’s esophagus in the general population: an endoscopic study. Gastroenterology. 2005;129(6):1825.
13. Sharma P and Sidorenko EI. Are screening and surveillance for Barrett’s oesophagus really worthwhile? Gut 2005; 54 Suppl 1: i27-32.
14. Dulai GS, Guha S, Kahn KL, et al. Preoperative prevalence of Barrett’s esophagus in esophageal adenocarcinoma: a systematic review. Gastroenterology 2002; 122 (1): 26-33.
15. Sampliner RE. The practice parameters committee of the American College of Gastroenterology. Practice guidelines on the diagnosis, surveillance, and therapy of Barrett’s esophagus. Am J Gastroenterol 1998; 93 (7): 1028-1032.
16. Sharma P, McQuaid K, Dent J, et al. A critical review of the diagnosis and management of Barrett’s esophagus: the AGA Chicago Workshop. Gastroenterology 2004; 127 (1): 310-330.
17. Gerson LB, Groeneveld PW, and Triadafilopoulos G. Cost-effectiveness model of endoscopic screening and surveillance in patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2004; 2(10): 868-79.
18. American Gastroenterological Association, Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology. 2011;140(3):1084.
19. Reid BJ, Levine DS, Longton G, et al. Predictors of progression to cancer in Barrett’s esophagus: baseline histology and flow cytometry identify low- and high-risk patient subsets. Am J Gastroenterol. 2000;95(7):1669.
20. Verbeek RE, van Oijen MG, ten Kate FJ, et al. Surveillance and follow-up strategies in patients with high-grade dysplasia in Barrett’s esophagus: a Dutch population-based study. Am J Gastroenterol. 2012;107(4):534.
21. Retrieved from September 2013. http://www.barrx.com/healthcare-professionals/why-treat-barretts-esophagus.php.
22. Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med. 2009 May 28;360(22):2277-88.
23. Shaheen NJ, Overholt BF, Sampliner RE,et al. Durability of radiofrequency ablation in Barrett’s esophagus with dysplasia. Gastroenterology. 2011 Aug;141(2):460-8
24. Fleischer DE, Overholt BF, Sharma VK,et al. Endoscopic radiofrequency ablation for Barrett’s esophagus: 5-year outcomes from a prospective multicenter trial. Endoscopy. 2010 Oct;42(10):781-9.
25. Manner H, May A, Pech O, Gossner L, et al. Early Barrett’s carcinoma with “low-risk” submucosal invasion: long-term results of endoscopic resection with a curative intent. Am J Gastroenterol. 2008;103(10):2589.
26. Pouw RE, Wirths K, Eisendrath P, et al. Efficacy of radiofrequency ablation combined with endoscopic resection for barrett’s esophagus with early neoplasia. Clin Gastroenterol Hepatol. 2010 Jan;8(1):23-9.
27. Ganz RA, Overholt BF, Sharma VK,et al. Circumferential ablation of Barrett’s esophagus that contains high-grade dysplasia: a U.S. Multicenter Registry. Gastrointest Endosc. 2008 Jul;68(1):35-40.
28. ASGE Standards of Practice Committee, Evans JA, Early DS, Fukami N,et al. The role of endoscopy in Barrett’s esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc. 2012 Dec;76(6):1087-94
29. Pouw RE, Gondrie JJ, Rygiel AM, et al. Properties of the neosquamous epithelium after radiofrequency ablation of Barrett’s esophagus containing neoplasia. Am J Gastroenterol. 2009;104(6):1366.
30. Gray NA, Odze RD, Spechler SJ Buried metaplasia after endoscopic ablation of Barrett’s esophagus: a systematic review. Am J Gastroenterol. 2011;106(11):1899.