
Winter 2013 - Vol. 8, No. 4
The Gut Flora
Christopher Shih, M.D., F.A.C.G.
Regional Gastroenterology Associates of Lancaster
INTRODUCTION
There have been many recent articles about the importance of the gut flora and their impact on, and association with, both disease and wellness. Each of us carries an estimated 100 trillion microorganisms in our intestines, a number that is roughly ten times higher than the number of human cells in our bodies.1 Bacteria make up most of the flora of the colon, and approximately two-thirds of the dry mass of feces. It is estimated that around 500 different species of bacteria live in the gut, with some protozoa and fungi as well, although the latter are not as well understood. Until recently, the importance of the gut flora has largely been ignored, or at least only appreciated in certain specific disorders, such as small bowel bacterial overgrowth. There is still much that we do not understand, but scientific advances over the past few years have demonstrated that the gut flora can profoundly affect everything from irritable bowel syndrome and inflammatory bowel disease to mental health, obesity, and infection with Clostridium difficile (CDI).
ROLE OF THE GUT FLORA
Figure 1: Realistic rendering of gut flora bacteria. Used with permission from Syontix.com.
Infants are born with sterile guts, and there are no bacterial species or microorganisms present in the digestive tract immediately after birth. However, colonization occurs shortly after birth from a number of sources: fecal transmission during vaginal delivery, nursing and general contact with the mother, and exposure to air and other household and community contacts. Babies born by caesarian section may have delayed colonization compared with babies delivered vaginally, but eventually they catch up. Within a few days after birth, bacterial colonies reach numbers in the billions, populated by Bifidobacterium, Bacteroides, Clostridium, and Ruminococcus species.2
Like all symbiotic relationships, our gut flora serve many important functions, including:
- digesting unutilized energy substrates,
- stimulating the immune system and cell growth,
- synthesizing vitamins,
- repressing the overgrowth of harmful microorganisms.
Bacterial overgrowth in the small bowel, which interferes with the normal functions of the native flora, can cause malabsorption of important nutrients, but not usually of folic acid, which is produced by the excess bacteria in large quantities. Harmful organisms like C. difficile are normally unable to flourish due to competition from beneficial bacteria, but when this balance is disrupted, as can occur with antibiotic use, pseudomembranous colitis can result.
Figure 2: Functions of the intestinal flora. (A) Bacteria density increases in the jejunum/ileum from the stomach and duodenum, and in the large intestine, colon-residing bacteria achieve the highest cell densities recorded for any ecosystem. The most common anaerobic and aerobic genera are listed. (B) Commensal bacteria exert a miscellany of protective, structural and metabolic effects on the intestinal mucosa. Reprinted by permission from Macmillan Publishers Ltd: EMBO Reports 7, 688 – 693 (2006).
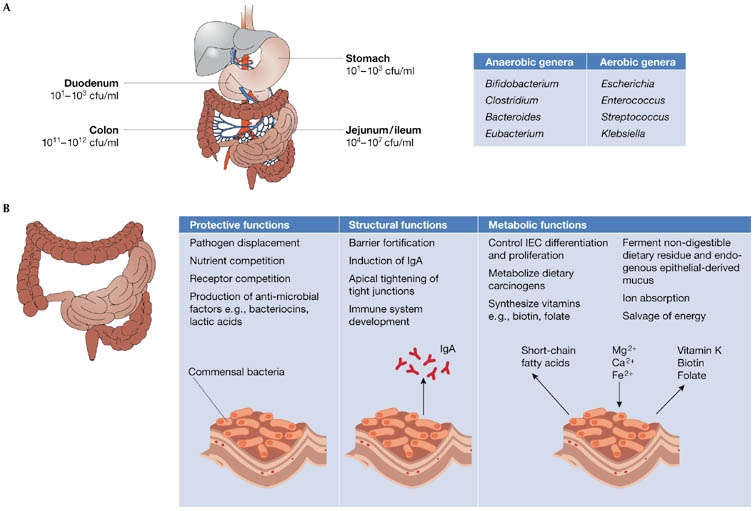
There are numerous examples of the gut flora’s important role in immunity: studies have shown that children with severe allergies have different bacterial compositions than children without allergies.3 In vitro studies have demonstrated that the gut flora are critical to the proper development of the immune system at a molecular level.4 Recent evidence suggests that the gut bacteria play an important role in the expression of toll-like receptors (TLRs) in the intestines, molecules that help repair damage and injuries to the digestive system. TLRs, along with nucleotide-binding oligomerization domain/caspase recruitment domain isoforms (NOD/CARD), make up the “pattern-recognition receptor” proteins, which allow the gut to distinguish between commensal, helpful organisms and pathogenic, harmful organisms. They have been identified as strong familial risk factors for the development of inflammatory bowel disease, which encompasses ulcerative colitis and Crohn’s disease. Interestingly, inflammatory bowel disease, while relatively common in developed countries, is virtually unreported in third-world nations. As a result, it is postulated that excessive hygiene and relative lack of exposure to microorganisms lead to faulty immune system development, and eventually to autoimmune responses that result in inflammation.5 Further, certain bacterial strains have even been associated with accelerated tumor growth, while still others have been shown to suppress it.
HARMFUL AND BENEFICIAL ALTERATIONS IN GUT FLORA
Clinically, these advances in understanding have resulted in a number of important and useful developments. Antibiotics, by altering the gut flora, can cause diarrhea in a number of ways, independent of whether or not C. difficile infection (CDI) is present. The mechanism for what we call “antibiotic-associated diarrhea” may be a disruption in the fecal flora leading to an imbalance between beneficial and harmful bacteria, which may also be one of the mechanisms in a number of other conditions including irritable bowel syndrome. Conversely, probiotics are thought to restore a healthy balance between beneficial and harmful bacteria in the gut, and studies have shown a clear benefit of certain probiotic strains in disorders ranging from irritable bowel syndrome to inflammatory bowel disease to the prevention of superinfections like C. difficile. The latter has become increasingly difficult to treat over the years, in part because of the development of resistant and hypervirulent strains.6-8 Probiotics are not always effective, which has led to consideration of fecal microbiota transplantation (FMT), which has emerged in recent years as a promising adjunct to the treatment of refractory CDI by rapidly and massively altering the gut flora.9 The theory behind FMT, similar to that of probiotics, is that the healthy donor’s stool can replace the diseased host’s stool and thus restore a proper balance to the gut flora. This approach is not new and has been practiced for years in the prevention of Salmonella infection in chickens.10
Figure 3: CDI causes severe diarrhea, intestinal inflammation and cell death as a result of toxin-mediated infection with the pathogenic bacteria. Patients with CDI are typically treated with antibiotics, which not only kill the pathogenic C. difficile but also exhibit activity against the dominant colonic microbiota phyla. Incomplete antibiotic eradication of C. difficile can result in recurrent CDIs. Transplantation of fecal microbiota from a healthy donor into an individual with CDI can restore the healthy gut microbiota in the patient's diseased colon, leading to resolution of symptoms. Reprinted by permission from Macmillan Publishers Ltd: Nature Reviews Gastroenterology & Hepatology 9, 88-96 (2012).
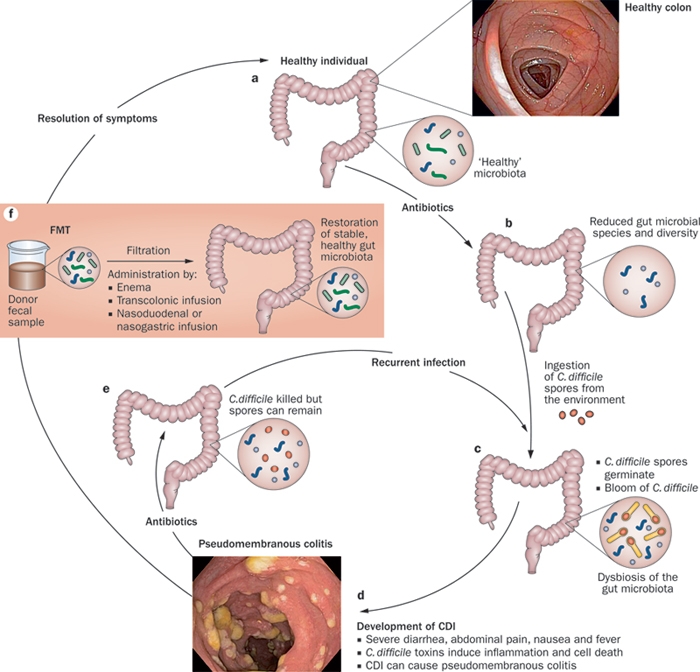
A randomized controlled trial published in the New England Journal of Medicine in 2013 proved FMT to be highly effective in treating recurrent CDI and more effective than vancomycin alone.11 In fact the study was stopped prematurely because the cure rate in the group that received FMT was 94%, compared with 31% in the group treated with vancomycin. Physically, the procedure involves single or multiple infusions of stool originating from a healthy donor, and they can be transferred to the recipient via enema, nasogastric tube, or colonoscopy. The recipient typically undergoes broad-spectrum antibiotic wipe-out of their existing flora prior to transplantation. It is not necessary that the donor and recipient be related, although in practice this is often the case. Occasionally multiple treatments are required; however, studies have shown that the majority of patients achieve eradication of their C. difficile with only one infusion.12
Because of concerns regarding aesthetic issues with FMT, scientists in Canada have recently reported administering oral gel capsules filled with fecal microbes for treatment of CDI.13 These oral capsules successfully resolved all but one of the infections. More studies are needed, but this promising avenue may one day make fecal transplant much simpler and more accessible. Research has even been conducted on exploring autologous fecal transplants, where the patient’s own stool is collected and stored prior to antibiotic treatment. If the patient then develops CDI, this previously collected stool can be delivered to his or her own colon to produce the same effect.14 FMT is deemed to be safe, as there have been no infectious reports in over 350 cases in the literature. And although it is only FDA-approved for treatment of refractory CDI, mounting evidence, both anecdotal and reported, shows promise in conditions that include inflammatory bowel disease, irritable bowel syndrome, and neurological disorders such as Parkinson’s disease.15
IMPLICATIONS FOR OTHER ORGAN SYSTEMS
The fact that bacteriotherapy has potential in neurological disorders underscores the critical relevance of the brain-gut axis. There is no question that our minds have a profound influence on our gut function, and this has been proven in both bench and clinical research, but recent evidence suggests that this is not necessarily a one-way street. The composition of our gut flora can actually affect our mental health, well-being, and even appetite. A recent report demonstrated that obesity in mice can be influenced by the gut flora they are fed.16 When mice without their own native flora were fed bacteria from an obese twin, they got fat. When they were fed bacteria from a non-obese twin, they stayed slim. Furthermore, the study showed that the flora from lean mice could “out-compete” the flora from obese mice: when the mice were mixed and ate each other’s feces, the obese mice became leaner. Additionally, the study showed that mice eating a high-fat diet retained their obese flora, but when they were switched to a leaner diet of fruits and vegetables, the lean gut flora took over. It is notable that for decades we have known that feeding antibiotics to farm animals promotes weight gain, possibly by altering their gut flora in some way.
In other research, our gut bacteria have been implicated in psychiatric disorders like anxiety, schizophrenia, autism, and obsessive-compulsive disorder. One recent study demonstrated that levels of HPHPA, the chemical byproduct of Clostridia bacterial species, were higher in patients with autism and schizophrenia.17
FMT in Lancaster
These fascinating advances hold tremendous promise for the future treatment of not only digestive disorders, but also a wide range of important diseases such as obesity, which is reaching epidemic proportions. The physicians of Regional Gastroenterology Associates of Lancaster anticipate being able to offer FMT for refractory CDI in Lancaster in the near future, and as our understanding of this field grows, we hope to acquire more and more tools to better serve our patients, and to potentially cure their disabling and often deadly diseases.
REFERENCES
1. Sears, CL. "A Dynamic Partnership: Celebrating Our Gut Flora." Anaerobe 11(5) (2005): 247-251.
2. Guarner, F. et al. "Gut Flora in Health and Disease." Lancet 361 (9356) (2003): 512-519.
3. Bjorjsten, B. et al. "Allergy Development and the Intestinal Microflora During the First Year of Life." J. Allergy Clin. Immunol. 108(4) (2001): 516-520.
4. Keeley, J. Good Bacteria Trigger Proteins to Protect the Gut. Howard Hughes Medical Institute. 2004. 9 January 2007.
5. Guarner, F. et al. "Role of Bacteria in Experimental Colitis." Best Pract Res Clin Gastroenterol 17 (5) (2003): 793-804.
6. Ortiz-Lucas, M. et al. "Effect of Probiotic Species on Irritable Bowel Syndrome Symptoms: Up to Date Meta-Analysis." Rev Esp Enferm Dig 105(1) (2013): 19-36.
7. Hahm, K. et al. "High Concentrated Probiotics Improve Inflammatory Bowel Diseases Better Than Commercial Concentration Of Probiotics." Journal of Food & Drug Analysis 20 (2012): 292-295.
8. Surawicz, CM. "Role of Probiotics in Antibiotic-Associated Diarrhea, Clostridium difficile-Associated Diarrhea, and Recurrent Clostridium difficile-Associated Diarrhea." J Clin Gastroenterol 42 Suppl 2 (2008): S64.
9. Bakken, J. et al. "Treating Clostridium difficile Infection with Fecal Microbiota Transplantation." Clinical Gastroenterology and Hepatology 9(12 (2011): 1044-1049.
10. Nurmi, E. et al. "New Aspects of Salmonella Infection in Broiler Production." Nature 241 (5386) (1973): 210-211.
11. van Nood, E. et al. "Duodenal Infusion of Donor Feces for Recurrent Clostridium difficile." N Engl J Med 368 (2013): 407-415.
12. Kelly, CR et al. "Fecal Microbiota Transplantation for Relapsing Clostridium difficile Infection in 26 Patients." J Clin Gastroenterol 46(2) (2012): 145-149.
13. Louie, T. et al. "Fecal microbiome transplantation via oral fecal microbial capsules for recurrent Clostridium difficile infection." IDWeek 2013 (2013): Abstract 89.
14. Martin, WJ. Encapsulated Medicine for Iatrogenic Diseases. Great Britain: Patent GB0916335.3. 2009.
15. Borody, TJ et al. "Fecal Microbiota Transplantation and Emerging Applications." Nat Rev Gastroenterol Hepatol 9(2) (2011): 88-96.
16. Ridaura, V. et al. "Gut Microbiota from Twins Discordant for Obesity Modulate Metabolism in Mice." September 2013. Science Vol. 341 no. 6150. October 2013.
17. Shaw, W. "Increased urinary excretion of a 3-(3-hydroxyphenyl)-3-hydroxypropionic acid (HPHPA), an abnormal phenylalanine metabolite of Clostridia spp. in the gastrointestinal tract, in urine samples from patients with autism and schizophrenia." Nutr Neurosci 13(3) (2010): 135-143.