
Fall 2013 - Vol. 8, No. 3
A Call for Participants: Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) for Hyperlipidemia
Tina Davis, MSN, CRNP, Rolf Andersen, M.D., FACC, and Jose Ibarra, M.D., FACC
The Heart Group of Lancaster General Health
INTRODUCTION
Hyperlipidemia is a major contributing factor in the development of cardiovascular disease (CVD), causing nearly 600,000 deaths a year in the United States alone. Cardiovascular events occur in 2 million Americans yearly, accounting for one quarter of inpatient hospital costs.1 Since reduction in low-density lipoprotein cholesterol (LDL-C) results in reduced cardiovascular morbidity and mortality, treatment of CVD has mainly focused on managing hyperlipidemia.2 Still, heart disease remains the leading killer of Americans despite major gains over the past half century due to the use of statins and encouragement of therapeutic lifestyle changes (TLC) such as diet, exercise, weight loss, and smoking cessation.
THE EFFECT OF LIFESTYLE CHANGES
These lifestyle changes are partially responsible for a notable reduction in the prevalence of elevated LDL-C levels: from 57% of Americans in the 1970s, to 27% today.1 For example, in the Profile Intervention Trial, a diet low in saturated fat, plus the addition of plant stanols/sterols, nuts, soy and soluble fiber, resulted in a 30% reduction in LDL.3 More recently, the Primary Prevention of Cardiovascular Disease with the Mediterranean Diet trial revealed a 30% reduction in CV events in subjects who followed a Mediterranean diet high in unsaturated fats.4 Given that not all hyperlipidemic individuals achieve LDL-C goals with TLC alone, and that the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP) guidelines recommend pharmacological therapy for anyone who has cardiovascular disease, researchers continue to seek an improved therapeutic solution.
THE ROLE OF STATINS
Statins are currently the gold standard for primary and secondary prevention of cardiovascular disease. Although statins significantly reduce LDL-C, and achieve a relative risk reduction for CV events of up to 42%, they do not eliminate the risk of such events. Moreover, the shortcomings of statins include potential adverse effects, drug interactions, and intolerability. In clinical trials of statins, 10-15 % of subjects experience myalgias and 0.1 to 0.2% develop statin-induced myopathies.6,7 Fatal rhabdomyolysis, a far more serious adverse effect, has been reported at the rate of less than 1 death for every 1 million individuals receiving statins.8
Another potential adverse effect is elevated hepatic transaminases occurring in up to 1% of individuals. This finding is usually related to the statin dose, and fortunately is easily reversed.9 Finally, more recent information has revealed that subjects who are prescribed statin therapy and have risk factors for diabetes have a 10-22% risk (depending on the particular statin taken) of developing high fasting glucose levels and/or diabetes mellitus type 2. An additional dilemma is that the NCEP ATP III guidelines recommend more aggressive LDL-C reduction, making it difficult for patients to achieve LDL-C goals below 70 mg/dL and 100 mg/dL. Additional high dose statins and combination therapy are required to reach these goals, placing patients at higher risk for adverse effects and intolerability. Overall, despite substantial improvements in treatment agents, 16-53% of individuals worldwide do not meet their optimal LDL-C goals.10
LDL-C goals are even more complicated for patients with Familial Hypercholesterolemia (FH) who have a more severe form of dyslipidemia characterized by abnormalities in the gene for the LDL receptor. With baseline LDL-C levels of 190 mg/dL or above for heterozygous FH, and 500 mg/dL for homozygous FH, up to 80% of FH patients do not reach their LDL-C goals10 and may require lipid apheresis treatments, which bring their own challenges. The treatments are invasive, time consuming (3-4 hours weekly or biweekly), costly, not universally available, typically require an AV fistula, and have potential side effects.
PCSK9 ANTIBODY: THE PHYSIOLOGICAL BASIS
In the last 4 decades, pharmacological technology has progressed dramatically, and medications are being developed that lower LDL-C through intestinal and hepatic pathways. In 2003, the Atherosclerosis Risk in Communities (ARIC) Study revealed PCSK9 “gain of function” mutations in two French families that resulted in a form of autosomal Familial Hypercholesterolemia with severely elevated LDL-C levels11 and an even higher than usual risk for CHD.12 On the other hand, in 2005 a causative relationship was identified between “loss of function” mutations in PCSK9 and low levels of LDL-C that were associated with a 47-88% reduction in the risk of CHD.13 The discovery of a PCSK9 deficiency mutation that resulted in such incredible CHD risk reduction heightened interest in this protein as a therapeutic target, and initiated intensive research efforts.
Monoclonal antibody PCSK9 inhibitors show very promising results in reducing LDL-C, ApoB and Non-HDL with great tolerability and projected reduction in CHD risk. PCSK9 is known to affect circulating LDL-C levels by binding to LDL receptors on hepatic cells so that fewer are available to remove LDL-C from the blood. (In contrast, traditional LDL-lowering therapies such as statins actually stimulate the production of PCSK9, thus limiting their ability to lower LDL-C.) Inhibiting the PCSK9 pathway is therefore a potentially novel mechanism for lowering LDL-C (Fig. I).14
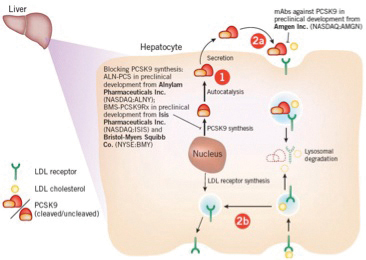
Currently six companies have developed monoclonal antibody PSCK9 inhibitors that are in various stages of clinical trials. No outcome studies are completed yet, but the initial Phase 1 and Phase 2 trials reveal excellent efficacy, with average reductions in LDL-C of 71%, and reductions in CHD risk of 47-88%. There have been very few adverse events. The preliminary results are so impressive that in a current NY Times article, Amgen stated that they “plan to make metric tons of its drug, more than any other biologic produced.”15 The article also notes that large pharmaceutical companies have already set up large factories to produce the new drug upon approval by the FDA.
THE PCSK9 TRIAL IN PRIMARY HYPERCHOLESTEROLEMIA – SANOFI
This Phase 2 Trial by Sanofi was a multicenter, prospective, double-blinded trial that investigated the safety and efficacy of SAR236553 (Sanofi’s designation for their monoclonal antibody PSCK9 inhibitor) in patients with hyperlipidemia despite having taken statins.
STUDY DESIGN
The Sanofi Phase 2 trial involved 92 patients who had LDL cholesterol levels of 100 mg/dl or higher after treatment with 10 mg of atorvastatin for at least 7 weeks. Patients were randomly assigned to one of three groups:
- Treatment group one received 8 weeks of treatment with 80 mg of atorvastatin daily plus SAR236553 once every 2 weeks;
- Treatment group two received 10 mg of atorvastatin daily plus SAR236553 once every 2 weeks;
- The control group received 80 mg of atorvastatin daily plus placebo once every 2 weeks.
STUDY RESULTS
After 8 weeks, an LDL cholesterol level of less than 100 mg/dl was achieved by 100% of the patients who received SAR236553, and by 52% of those in the control group who received 80 mg of atorvastatin plus placebo. In addition, an LDL cholesterol levels of less than 70 mg/dl was achieved by approximately 90% of the patients who received SAR236553, but only by 17% of those who received 80 mg of atorvastatin plus placebo.
THE TRIAL AT LANCASTER GENERAL HOSPITAL
Dr. Rolf Anderson, medical director of The Heart Group Risk Factor Clinic at LGH, emphasizes that these improvements in therapy for patients with severe lipid abnormalities are the result of improved understanding of the basics of cholesterol metabolism. He finds it notable that the discovery by pharmaceutical companies of this novel therapeutic approach came from genetic testing of people with moderately low cholesterol levels and no heart disease. The recognition that there is so little heart disease in PCSK9-deficient people with extremely low cholesterol levels from birth, further emphasizes the importance of getting cholesterol treatment started as early as possible.
Lancaster General Hospital is one of 1,200 sites across 48 countries participating in the ODYSSEY Outcomes investigational clinical trial. The objective of the trial is to evaluate the ability of SAR236533 to reduce CV events in patients who recently experienced an ACS event, but have not reached the goals recommended by the ACC guidelinesl for these very high risk patients, despite intensive statin therapy or at a maximally tolerated dose.
Health care professionals interested in learning more about the study and whether their patients might qualify should call 717-544-1777. Information for participants may also be found at www.odysseytrials.com/web/about_odyssey_program.
REFERENCES
1. Kuklina EV, Carroll MD, Shaw KM, Hirsch R. Trends in high LDL cholesterol, cholesterol-lowering medication use, and dietary saturated-fat intake: United States, 1976–2010. NCHS data brief, no 117. Hyattsville, MD: National Center for Health Statistics. 2013.
2. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001; 285: 2486–2497
3. Jenkins DJ et al. JAMA. 2011 Aug 24;306(8):831-9.
4. Estruch R et al. NEJM 2013; 368:1279-1290.
5. Scandinaviansimvastatinsurvival (November 1994). “Randomized trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S)”. Lancet 344 (8934): 1383–9.
6. Marcello A & Pigna G. Diabetes Metab Syndr Obes. 2011; 4: 155-166.
7. Hamilton-Craig I. MJA. 2001: 175: 486–489.
8. Newman CB et al. Am J Cardiol. 2003; 92: 670–676.
9. Staffa JA et al. N Engl J Med. 2002; 346: 539–540.
10. Tobert JA. Am J Cardiol. 1988: 62: 28J–34J.
11. Giuseppe, DN. Res Rep in Clin Caridology. 2013:4; 77-84.
12. Abifadel M et al. Nat Genetics. 2003; 34:154-156.
13. Humphries SE et al. J. Med. Genet. 2006; 43:943-949.
14. www.nytimes.com/2013/07/10/health/rare-mutation-prompts-race-for-cholesterol-drug
15. Lambert et al. J Lipid Res. 2012 Dec;53(12):2515-24.
16. Sanofi Press Release (May 26, 2012) “Sanofi and Regeneron Announce Publication of Positive Phase 2 Results for Lipi-Lowering PCSK0 Antibody in The Lancet”.