 Click to Print Adobe PDF
Click to Print Adobe PDF
Winter 2012 - Vol.7, No.4
|
A Call for Participants:
Renal Denervation Therapy for Refractory Hypertension
Rupal P. Dumasia, M.D., F.A.C.C., F.S.C.A.I.,
Section Chief, Interventional Cardiology, The Heart Group of Lancaster General Health
Paul N. Casale, M.D., F.A.C.C.
Chief, Division of Cardiology and Medical Director of Cardiology, Lancaster General Hospital
John Briguglio, M.D.
Cardiovascular and Interventional Radiologist
Caution: Investigational device. Limited by federal (or United States) law to investigational use
|
   |
INTRODUCTION
Hypertension is a serious and growing global public health concern that affects an estimated 30–40% of the adult population in developed countries. Seventy-six million Americans have hypertension, which puts them at increased risk of premature death, kidney disease, and cardiovascular complications such as stroke, heart attack and heart failure.1 The potential for serious complications makes it critical for treating physicians to control hypertension over the long term and to maintain careful and regular follow up.
Individuals whose hypertension is treated but not effectively controlled have a 3X greater risk of cardiovascular events than individuals whose hypertension is controlled.2 Yet, despite the availability of numerous safe and effective pharmacological therapies, nearly one third of hypertensives are resistant to treatment and do not achieve guideline target values for blood pressure. These failures of the pharmacological approach are largely attributed to physicians’ inertia and patients’ non-adherence to and non-persistence with lifelong pharmacological therapy for a disease that is commonly asymptomatic until the late stages. Thus, the development of new ways of approaching the management of hypertension, especially those that could help overcome these problems, is a priority.
RENAL DENERVATION FOR HYPERTENSION; THE PHYSIOLOGICAL BASIS
Chronic elevation of sympathetic nervous system (SNS) activity has been identified by extensive preclinical and clinical studies as a common and influential factor in diseases such as hypertension, heart failure, and chronic kidney disease. Because the renal sympathetic nerves are a major contributor to the complex pathophysiology of elevated SNS activity (and consequently to hypertension), therapeutic denervation of the kidneys has proven effective in modulating elevated SNS activity. Denervation not only reduces sympathetic control of various kidney functions (renin release, sodium excretion, and renal blood flow), but also removes the renal afferent sympathetic contribution to the central nervous system component of hypertension. It is important to note that the kidneys maintain appropriate electrolyte and volume homeostasis despite being denervated, as demonstrated by the human kidney transplant experience.
The concept of therapeutic renal denervation is not new; it has been explored in man with complex procedures such as surgical nephrectomy and even radical surgical sympathectomy to treat established pathology including severe hypertension. What is new is the performance of renal denervation by a catheter approach, and it is this type of therapy that is currently under investigation by the FDA.
RENAL DENERVATION BY CATHETER
The Symplicity™i catheter (Figure 1) is introduced using standard interventional techniques via the femoral artery, and is positioned in the renal artery under fluoroscopic guidance. The electrode is radiopaque, and to minimize thermal damage to the renal artery wall, the design allows continuous blood flow during the treatment which provides cooling of the artery wall. The catheter is torquable, so the user can easily rotate the catheter to treat different segments of the vessel.
Figure 1: The Simplicity Catheter
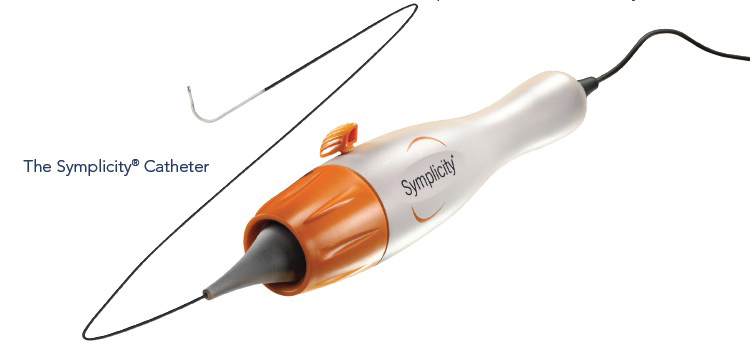
Figure 2
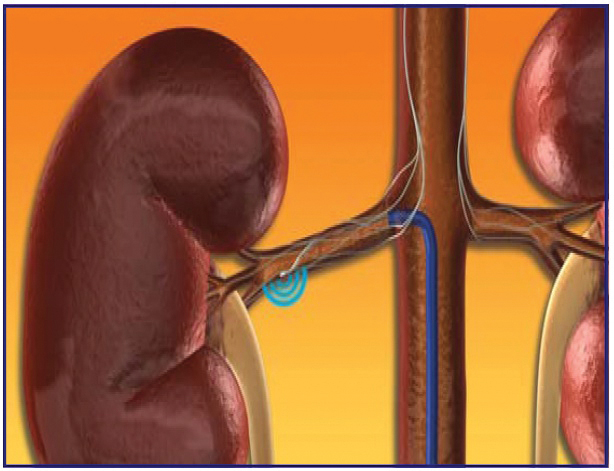
The treatment involves delivery of relatively low-power and precisely focused Radio Frequency bursts of approximately 8W through the wall of the renal artery to disrupt the surrounding renal nerves lying in the adventitia. The procedure does not involve a permanent implant.
THE SYMPLICITY HTN-3 CLINICAL TRIAL
The current Symplicity HTN-3 clinical trial is an international, multi-center, prospective, randomized, controlled, single-blinded investigational study of the safety and effectiveness of renal denervation therapy in patients with hypertension that is resistant to treatment.
Study design
Eligible subjects are blinded to their randomized assignment to one of two groups: a) Treatment Group: Subjects are treated with the renal denervation procedure and are maintained on baseline anti-hypertensive medications with no changes for 6 months. b) Control Group: Subjects are maintained on baseline anti-hypertensive medications with no changes for 6 months, after which, if eligible, they will have the option of being treated with the renal denervation procedure.
SYMPLICITY HTN 2
In an earlier trial that has already been completed, one hundred and six (106) patients at 24 participating centers were randomly allocated in a 1:1 ratio to undergo renal denervation with previous treatment or to maintain previous treatment alone. At baseline, the randomized treatment and control patients had similar high blood pressures (178/97 mm Hg and 178/98 mm Hg, respectively), despite both receiving an average daily regimen of five antihypertensive medications. At 6 months of follow-up, patients in the control arm of the study were offered renal denervation following assessment of the trial`s primary endpoint. 46 control patients crossed over (35 per protocol and 9 not per protocol; 2 were lost to follow-up).
At 6 months the need for hospitalization was similar in both groups. The treatment group had 3 hospitalizations for hypertensive emergencies unrelated to medication noncompliance, 1 for a hypertensive emergency related to cessation of clonidine, 1 for an episode of nausea/edema, 1 for a TIA, 1 for a hypotensive episode, and 1 for insertion of a coronary stent. The control group had 2 hospitalizations for hypertensive emergency unrelated to medication noncomplicance, 2 for TIAs, and 1 for insertion of a coronary stent.
18-MONTH DATA
Eighteen months data announced by Medtronic, the study sponsor, confirmed the intermediate term safety of renal denervation with the Symplicity™ renal denervation system, as there were no device-related serious adverse events and no newly reported vascular complications from 12-18 months.
The average number of medications for patients in this trial did not change during this period of follow-up. At 18 months, the Symplicity™ renal denervation system continues to provide superior and sustained blood pressure reduction. The patients originally randomized to renal denervation had a drop in BP of 32/12 mm Hg. The crossover patients (those who received RDN 6 months after originally being randomized to control) had a BP drop of 28/14 mm Hg. Pulse pressure improved significantly both in the group that received initial treatment with the Symplicity™ renal denervation system (-20 mm Hg from baseline; p<0.01), and in the crossover group (-18 mm Hg from baseline; p<0.01). Since pulse pressure may have predictive value in terms of cardiovascular complications, especially in older patients, it may be important to evaluate changes in pulse pressure as well as systolic and diastolic blood pressure when assessing the efficacy of antihypertensive therapy.
THE TRIAL AT LANCASTER GENERAL HOSPITAL
Lancaster General Hospital is one of 90 participating sites across the country in the Symplicity HTN-3 investigational clinical trial, and one of three in Southeastern PA. This trial will enroll people between the ages of 18 and 80 with treatment-resistant hypertension, and with a systolic blood pressure of more than 160 mmHg.
Health care professionals interested in learning more about the study and whether their patients might qualify should call 717-544-5951. Information for participants may also be found at www.SymplifyBPtrial.com.
References
1.Egan, Brent M., et al. Uncontrolled and apparent treatment resistant hypertension in the United States, 1988-2008. Circulation 2011; 124. 9: 1046-1058.
2.Doumas, Michael, et al. Benefits from treatment and control of patients with resistant hypertension. International Journal of Hypertension 2011 (2011) Article ID 318549, 8 pages, 2011. doi:10.4061/2011/318549
Note
i.Symplicity is a trademark of Medtronic Inc. and is registered in one or more countries of the world.