 Click to Print Adobe PDF
Click to Print Adobe PDF
Fall 2010 - Vol.5, No.3
Controversies in Screening Mammography
Nitin K. Tanna, M.D.
Lancaster Radiology Associates, Ltd.
|
 |
INTRODUCTION
When the United States Preventive Service Task Force (USPSTF) issued another round of recommendations for screening mammography in November, 2009, it rekindled a controversy that has intermittently plagued this procedure for several decades. The report issued by this independent panel (see below) was meant to apply only to women with an average risk of breast cancer, and recommended biennial screening mammography only for women aged 50 to 74. It does not recommend routine mammographic screening in women outside this age range, though it does suggest that women under the age of 50 seek medical advice and consultation to determine if mammography is indicated.1 It also retracts recommendations for an annual clinical examination of the breast (CBE), and monthly self breast examination (BSE).1
These recommendations have created enormous controversy and confusion in the medical community, and among women in general. Virtually every professional society, including the American Cancer Society, the American College of Surgeons, the American College of Obstetrics and Gynecology, the Society of Breast Imaging, Susan G. Komen for Cure, the Avon Foundation for Women, and the Mayo Clinic have condemned these recommendations.2 The American Society of Family Physicians is one of the few that have endorsed them.3 Even the US Secretary of Health and Human Services, Kathleen Sebelius, has told women to ignore the USPSTF recommendations and to follow the American Cancer Society’s guidelines to be screened every year.2 These recommendations were also rejected by Congress as it debated and approved the legislation for health care reform.
BACKGROUND
The USPSTF is a panel appointed by the Agency for Health Care Research and Quality of the Department of Health and Human Services, but it operates independently without any oversight. It is comprised of sixteen members, mostly epidemiologists and biostatisticians. While there are a few physicians on the committee, there are no individuals with particular expertise in the diagnosis or treatment of breast cancer, such as radiologists, oncologists, pathologists, or surgeons. In fact, when Daniel Kopans MD, Professor of Radiology at Harvard University, Massachusetts General Hospital, and the Avon Breast Center, and a world renowned authority on screening mammography, heard of the possible meetings of the task force, he emailed the committee and offered to assist them.2 The committee did not respond to his email.
The committee did not introduce any new scientific research, but essentially used the same randomized controlled trials used in its 2002 recommendations to develop an entirely different set of conclusions. The committee also spent considerable time on computerized modeling of the risk/benefit analysis of screening mammography. Virtually the only addition to the 2002 data base was an interim study from the United Kingdom which showed only a 15 % reduction in mortality from screening among women in the 40-48 age groups.4 According to most experts on breast imaging, this study was flawed in its design and randomization process and relied exclusively on one-view mammography examinations, a method that is a significant deviation from the standard and well accepted two-view examination of each breast. (Personal discussion with Dr. Edward Sickles, Professor Emeritus of Radiology, Breast Imaging Section, University of California School of Medicine, San Francisco).
Other randomized control trials, and particularly data from multiple trials in Sweden, show much better outcomes for screening in this age group. The Swedish data are some of the best accumulated in a randomized controlled fashion over nearly four decades, and they show a nearly 30-45% decrease in mortality in the screened groups compared with the unscreened groups. 5 6,7,8,9, Much of these data were omitted in the USPSTF analysis. Recent updates, with additional mortality data added to the original Swedish data set, may show a nearly 65 % reduction in mortality from breast cancer. (Unpublished data made available in conversation with Dr. Lazlo Tabar). Multiple other controlled trials, including the original HIP (Health Insurance Plan of Greater New York, 1963-1968), the Edinburgh trials,10 the British Columbia study11 and various Swedish and Danish Trials, 5 6,7,8,9, as well as the nearly 30% decrease in mortality from breast cancer among the 50% of US women who are compliant with current screening guidelines, all affirm the need for regular screening mammography in women. Furthermore, the USPSTF recommendations do not acknowledge the ever growing and evolving data on the biology and therapy of breast cancer, nor do they consider the advanced imaging tools now available, particularly digital mammography which is especially helpful in assessing the dense breast parenchyma of women under the age of 50. None of the trials to date have been performed with the use of digital mammography.
Some of the key reports often cited by opponents of screening mammography are the various versions of the Canadian National Breast Cancer Screening Studies (CNBCSS), which included one in the age group from 40-49 years, a separate longer term follow-up study in the same cohort, and another study in the age group from 50-59 years. Since these studies found no significant benefit for mammography,12,13,14 their inclusion in any outcomes modeling or meta-analysis skews the results against screening.
Importantly, none of those large Canadian studies was a truly randomized controlled trial, since patients were assigned to the screening or no-screening groups only after a physical examination of the breasts. Since women with palpable masses were selectively assigned to the screening cohort, it contained 4X as many advanced cancers as the non-screened group. This difference inevitably altered the mortality statistics, which compromised the parameter by which the effectiveness of screening mammography was assessed. Errors in certification of the cause of death were also raised by the authors in the first study, a fact that was even noted by the authors of the paper.12 Compliance among the women in these studies was also a problem, with nearly 20% of those assigned to regular screening not getting these examinations over a 4-5 year period, and many women assigned to the no screening cohort getting screening mammograms outside the study. Questions have also been raised about the quality of mammographic examination, the skills of the interpreting physicians, design study, randomization, contamination of controls, follow-up, and ascertainment, thus further eroding the conclusions reached in this study.15
SCREENING MAMMOGRAPHY
Analog screening mammography is a limited two-view examination of each breast, and is only advised for women who are completely asymptomatic. With digital mammography technology, the clarity of the images is superb, and the benefits of digital mammography are the greatest for younger women with dense breast tissue where analog examinations have limitations. (Figure 1) Mammography has the lowest radiation exposure of all radiographic examinations and even this amount is very tightly regulated by the Mammography Quality Standards Act, which is enforced by the Food and Drug Administration. Moreover, digital mammography results in a further decrease in radiation exposure to the glandular tissue by nearly 22% per view.16 These differences were not considered by the USPSTF when they emphasized the potential harm of screening mammography related to radiation exposure.

Figure 1a: Analog (film screen) right mediolateral oblique view (RMLO) on a patent with very dense breast parenchyma. The film screen mammogram was performed at an outside institution and provided to us as part of further workup and biopsy.
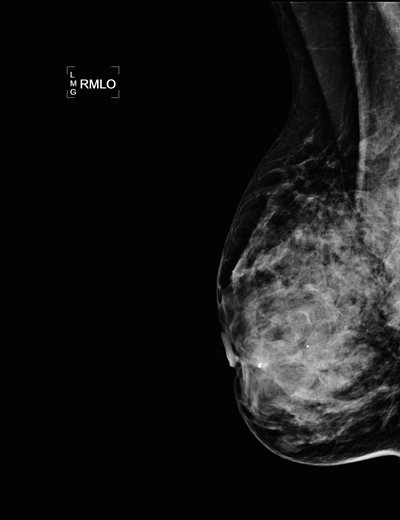
Figure 1b: Digital RMLO view on the same patient after a needle core biopsy and placement of clip in anterior right breast. Note the markedly improved tissue penetration, contrast, and better delineation of skin and subcutaneous tissues.
As noted earlier, the task force guidelines do not recommend routine screening mammography for women under the age of 50 with average risk. However, it is the view of most breast cancer experts that these are the women who most need to be screened regularly. (The Department of Health and Human Services deleted these new recommendations from its website within months after their initial publication.) Between 75 -85% of breast cancers are detected in women with no risk factors, and multiple studies have shown there is a substantial incidence of breast cancer in women under 50. Of the nearly 40,000 annual deaths from breast cancer, 18 % of the women were diagnosed in their 40’s.17 At Lancaster General Health, an average of 20 % of the breast cancers diagnosed in the 3 years 2007-2009 were diagnosed in women under the age of 50. (Table 1) Numerous studies have shown that these cancers in younger women are more aggressive, often presenting as multi-centric or multi-focal lesions. (Figure 5) The Swedish data have shown that clinically occult cancer in the 40-49 year old cohort has a 16 % chance of spreading to a lymph node versus a 7 % chance for a woman in her 50’s.18 Advanced cancers detected in the absence of regular screening mammography result in significant cost, morbidity, mortality, and alteration of quality of life. (Figure 2) These factors are not addressed in the new USPSTF guidelines. It is therefore critical to diagnose these preclinical tumors at their earliest stage and this can only be achieved with annual mammographic evaluation, supplemented by clinical and self breast examinations.
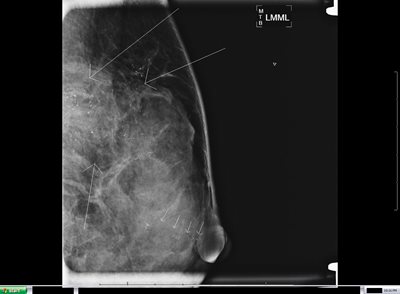
Figure 2a: Left breast magnification lateral view on a 46 year old patient who skipped several years of screening mammograms.
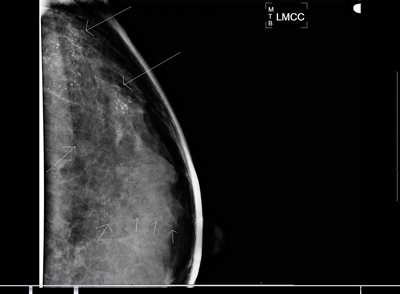
Figure 2b: Left breast magnification craniocaudal (CC) view on same patient. Arrows indicate extensive malignant type calcifications scattered throughout the breast and extending from chest wall to a duct that extends to the nipple (multiple arrows in alignment). After several years without mammography, this patient had extensive in situ and invasive ductal carcinoma with lymph node involvement. Because of the extent of disease, patient required mastectomy, neoadjuvant chemotherapy, and radiotherapy to chest wall and axilla.

Figure 3: Post biopsy lateral digital mammogram on a 59 year old patient with a very small 6 mm invasive ductal cancer with small radiopaque tissue marker clip (two arrows) but with a biopsy proven malignant left axillary lymph node(single arrow), despite the small size of the index lesion.
Nearly half of the breast cancers in the Lancaster County population are diagnosed in the 50-69 year age group, so the most prudent advice is to recommend the same annual screening schedule for these women as well. (Figure 3) The task force did not recommend screening for women over the age of 74, yet none of the randomized controlled trials they considered studied women over age 69, so there is no scientific evidence to support an argument against screening mammography in this age group. Furthermore, the selection of age 74 is arbitrary and without any scientific basis though it is a known fact that screening mammography in the National Health Service in the United Kingdom ends at 74. Again, our local numbers mirror the national figures (Table 1). Over a three year time frame, an average of 19.5% of the breast cancers we detected were in women 70-79 years of age, and another 11.3 % were in women over 80. Almost all of the previously mentioned professional societies support routine screening mammography as long as a woman has at least another 5-7 years of life. Of course, comorbidities need to be considered, and the decision must be individualized, but there is nothing magical about the age of 74 that should result in cessation of screening mammography (nor age 50 for starting it). As long as an older woman is willing and able to tolerate appropriate treatment for the cancer, mammography should be considered. (Figure 4)
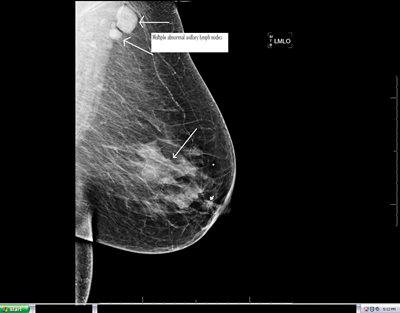
Figure 4a: Left MLO view on 79 year old, otherwise healthy woman with biopsy proven small cancer in anterior left breast(small arrowhead) and a much larger biopsy proven but radiographically occult malignancy (detected by breast MRI) in central left breast (large arrow) and multiple, biopsy proven, malignant lymph nodes in the left axilla (two arrows).
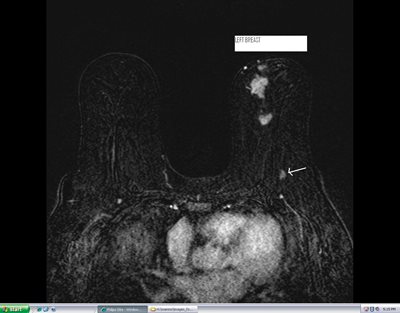
Figure 4b: Contrast enhanced breast MRI of both breasts on same patient shows multiple enhancing, biopsy proven, malignancies in the anterior half of left breast. MRI of the breast is extremely sensitive in detection of breast cancer. One of the lymph nodes in posterior left breast is also partially visualized on the breast MRI (arrow on posterior enhancing mass).
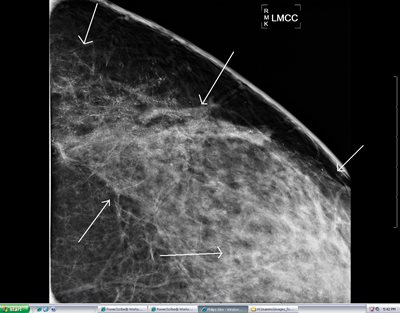
Figure 5: Magnification CC view on a 30 year old patient showing extensive malignant type calcifications in the left breast. This extensive clinically occult cancer was only diagnosed with the benefit of the digital mammogram.
It is also very important to stress that these guidelines apply only to asymptomatic women at no particular risk for breast cancer. A more aggressive screening approach would benefit: women with BRCA1 or BRCA2 mutations; a history of various syndromes and other genetic predispositions to breast cancer; and first degree relatives of these individuals, regardless of their genetic testing status. This approach would involve annual diagnostic mammograms and breast ultrasound examinations as needed, staggered every six months with annual Breast MRI. Women with chest wall radiation for other malignancies such as lymphoma also fall into this high risk group that requires very aggressive screening every six months.
The task force recommendation to omit the physical examination and breast self examination is also controversial. Most women with palpable masses are feeling benign conditions such as lumpy breast tissue, or benign conditions such as cysts or fibroadenomas. Though mammography does have its limitations, and a palpable mass occasionally represents a cancer that is mammographically occult, a combined detailed imaging workup of a palpable mass by a radiologist trained in breast imaging can make the chance of missing a malignancy <1%. Again, the patient with a palpable mass requires not a screening mammogram, but a diagnostic mammogram and ultrasound by a radiologist who specializes in breast imaging. Hence, the traditional recommendation of supplementing mammography with clinical examination and self examination of the breast is the most sensitive combination in a woman who is asymptomatic and has average risk factors.
The task force did appropriately emphasize the limitations of screening mammography. Once again, however, their data only included studies done in the era of analog film technology for screening examinations and not digital mammography. Mammography is not a perfect screening test, and its shortcomings need to be discussed with every patient. Inherent in every process that takes a three-dimensional object and creates a two-dimensional image is the visualization of overlapping normal structures that can mimic a true mass. Interpretation of these densities can often be resolved with additional views or ultrasound, which requires that the patient be recalled for further workup.
It is generally accepted that in dedicated breast centers no more than 10% of screened women are recalled, and at LGH our recall rate is below the national benchmark. On average, if 1000 women are screened, 80-100 will be recalled for additional views. 60 to 70 of these 100 will have the issue resolved with the additional workup and can return to routine screening. About 20 of the 100 will be asked to return for short-term follow-up in 6 months, 15 will be recommended for biopsy, and about 2-5 women will be diagnosed with breast cancer.17 Of course, these recalls and biopsies generate anxiety and incur costs, but in the opinion of most breast cancer experts, the USPSTF has overemphasized these concerns relative to the benefits.
Most women are aware of the limitations of mammography. Most breast biopsies are now performed by the percutaneous needle core technique and not with open surgical biopsy, which reduces the costs and morbidity of breast biopsy. About 90-95 percent of breast biopsies can be successfully performed percutaneously, a conclusion supported by a recent consensus panel of breast surgeons.19 At the slightest hint of a potential abnormality, most women want to undergo a percutaneous biopsy and get the reassurance that they do not have cancer. A 2004 JAMA article showed that 99% percent of patients studied accept the false positive risk of screening mammography and other cancer screening.20 63% of these women further believed that 500 or more false positive examinations are acceptable for each life saved.21
CONCLUSIONS
The recommendations issued by the USPSTF regarding screening mammography have not been adopted by the majority of professional societies or federal agencies. The USPSTF recommendations were also not meant to be used to determine reimbursement for screening mammography, but inevitably, a few states (such as cash-strapped California) are using these guidelines to allocate the already scarce resources in the state’s budget for cervical and breast cancer screening programs. New York, Florida, Illinois and Michigan have also attempted to modify the reimbursement for screening mammography based on these new recommendations.3
In response to these attempts, and given the nearly 210,000 invasive breast cancers diagnosed each year and the nearly 40,000 breast cancer deaths, the Department of Health and Human Services announced on July 14, 2010 that all insurance companies must pay for certain screening tests, such as screening mammography, for any woman over age 40. This announcement in essence reinforces the 2002 USPSTF recommendations, and rejects the new 2009 recommendations.
The current recommendations for annual screening mammograms starting at age 40 and continuing as long as the patient is willing and capable of tolerating the traditional treatment for breast cancer remains widely accepted despite the new USPSTF guidelines. Of course, treatment guidelines are being modified each year as newer data become available, and as we better understand the biology and natural history of breast cancer.
The task force recommendation to adequately inform the patient prior to the mammogram about the limitations of mammography and the potential need for additional testing is important. However, it is impractical for most physicians to have a 15 -30 minute discussion and analysis about the benefits and harms of screening during an office visit where there are a multitude of concerns. Moreover, it is clear that most women would rather undergo this testing despite knowing its limitations. We as physicians need to emphasize the importance of screening mammography. In Sweden, there is an 85 % compliance rate for screening mammography in women of eligible age, but in the United States the national compliance is on the order of 50 %. Recent demographic analysis of Lancaster County suggests a compliance rate with screening guidelines that is far lower - about 25%. With the new HHS ruling on payment for mammography, it only makes sense that preventive screening mammography needs to be increased. Only by achieving greater compliance with the widely accepted traditional guidelines can we hope to achieve improved outcomes in our patients.
REFERENCES
1 USPSTF, Screening for Breast Cancer: U. S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2009; 151(10):716-727.
2 Lowry, Fran. Top Mammography Experts Voice Outrage over New Breast Cancer Screening Recommendations. Medscape Medical News as Press Release, Radiological Society of North America (RSNA) 95th Scientific Assembly and Annual Meeting. December 3, 2009
3 Gibbons, Michael. Screening’s Defenders and Doubters. Advance for Imaging and Radiation Oncology. 2010; 20(6): 8-12.
4 Moss S M, Cuckle H, et al. Effect of mammographic screening from age 40 years on breast cancer mortality at 10 years’ follow-up: a randomized controlled trial. Lancet 2006; 368: 2053-2060
5 Anderson I, Janzon L. Reduced breast cancer mortality in women under 50: updated from Malmo Mammographic Screening Program. J Nat Cancer Inst Monogr 1997; 22: 63-67
6 Bjurstam N, Bjorneld L, et al. The Gothenburg Breast Screening Trial. Am Cancer Soc 2003; 97: 2387-2396
7 Tabar L, Vitak B, Chen HH, et al. The Swedish two county trial twenty years later: updated mortality results and new insights from long term follow up. Radiol Clin N Amer. 2000; 38: 625-651
8 Tabar L, Yen MF, Vitak B, Chen HH, Smith RA, Duff SW. Mammography service screening and mortality in breast cancer patients: 20 year follow up before and after introduction of screening. Lancet. 2003; 361(9367): 1405-1410.
9 Duffy SW, Tabar L, Chen HH et al. The Impact of Organized Mammography Service Screening on Breast Carcinoma Mortality in Seven Swedish Counties. Cancer. 2002; 95: 458-469
10 Alexander FE, Anderson TJ, Brown HK et al. 14 years follow up from the Edingurgh randomized trial of breast cancer screening. Lancet 1999; 353: 1903-1908.
11 Coldman A, Phillips N, Warren L, Kan L. Breast Cancer Mortality after screening mammography in British Columbia women. Internat J Cancer. 2007; 120(5): 1076-1080
12 Miller AB, Baines CJ, To T, Wall C. Canadian National Breast Screening Study: 1. Breast cancer detection and death rates among women aged 40-49 years. Canad Med Assoc J. 1992; 147(10): 1459-1476.
13 Miller AB, To T, Baines CJ, Wall C. The Canadian National Breast Screening Study- 1: Breast Cancer Mortality after 11 to 16 years of follow-up. Ann Intern Med. 2002; 137: 305-312
14 Miller AB, To T, Baines CJ, Wall C. Canadian National Breast Screening Study-2: 13 year Results of a Randomized Trial in Women Aged 50-59 Years. J Natl Cancer Inst 2000; 92: 1490-1499.
15 Baines CJ. The Canadian National Breast Screening Study: A perspective on Criticism. Ann Intern Med. 1994; 120: 326-334
16 Hendrick RE, Pisano ED, Averbukh A et al. Comparison of Acquisition Parameters and Breast Dose in Digital Mammography and Screen-Film Mammography in the American College of Radiology Imaging Network Digital Mammographic Imaging Screening Trial. AJR 2010; 194: 362-369
17 Berg WA. Benefits of Screening Mammography. JAMA 2010; 303(2):168-169.
18 Swedish Cancer Society and the Swedish National Board of Health and Welfare. Breast Cancer screening with mammography in women aged 40-49 years. Int J Cancer 1996; 68(6): 693-699.
19 Silverstein MJ, Recht A, Lagio MD, et al. Image-detected Breast Cancer: State-of-the-Art Diagnosis and Treatment (special report: consensus conference III). J Am Coll Surg. 2009; 209(4): 504-520.
20 Schwartz LM, Woloshin S, Fowler FJ, Welch HG. Enthusiasm for Cancer Screening in the United States. JAMA. 2004; 291: 71-78.
21 Schwartz LM, Woloshin S, Sox HC, et al. US women’s attitudes to false positive mammography results and detection of ductal carcinoma in situ: cross sectional survey. Br Med J. 2000; 320(7250): 1635-1640.