 Click to Print Adobe PDF
Click to Print Adobe PDF
Summer 2010 - Vol.5, No.2
Tick-Borne Illnesses, Part I: Lyme Disease, Anaplasmosis, and Babesiosis
Joseph M. Kontra, M.D.
Infection Specialists of Lancaster
|
 |
Wet spring morning grass
In silent meditation
A nascent tick quests
ABSTRACT
The arrival of spring brings to the Infectious Diseases specialist the challenge of an ever-expanding array of microbial diseases transmitted to humans through contact with ticks. While the causative agents of Lyme disease, Anaplasmosis, and Babesiosis are disparate, their similar clinical presentations, geographic distribution, and the possibility of co-transmission via a common vector warrant their consideration as coherent group of pathogens. Part I of this series will review the pertinent clinical, epidemiologic, biologic, and management issues of these three important infections.
LYME DISEASE
Historical Aspects
The first description of Lyme arthritis dates back to 1977, with a study of a series of Connecticut children thought to have juvenile rheumatoid arthritis. The seasonal occurrence, and geographic clustering of illness suggested an infectious etiology. 1 The spirochete nature of the Lyme disease pathogen was described years later by Dr. Willy Burgdorfer,2 after whom the causative agent Borrelia burgdorferi was named. Multiple tick vectors were later identified, and a burgeoning pandemic over the next two decades was observed. Lyme disease is now the most common tick borne infection in both the United States and Europe, and is a significant cause of disease in Asia.
Microbiology
B. burgdorferi (B.b.) is a fastidious flagellated spirochete bacterium. It joins a much older cadre of human pathogenic spirochetes, which includes Treponema pallidum (syphilis), Leptospira interrogans (leptospirosis), and the multiple Borrelia species that cause relapsing fever.
B. burgdorferi consists of three pathogenic species. B. burgdorferi sensu stricto is the sole cause of disease in the United States, and is the focus of this review. This species occurs in Europe as well, as do B. afzelii and B. garinii which also cause disease in Asia.
B. b. contains a small linear genome similar to other Borrelia species, and is efficiently suited for its obligate parasitic nature. While the organism lacks any of the classical virulence factors, such as lipopolysacharride or exotoxins, two key genes have been identified. Outer Surface Protein C (OspC) is required for host colonization and resistance to innate immunity. Products of a variable sequence locus (VlsE) are involved in resistance to the neutralizing antibodies of acquired immunity.3 A third gene, OspA, while not a virulence factor, was utilized as the target for the Lyme disease vaccine, and will be discussed later.
Tick Vectors and Life Cycle
Ixodes scapularis (Fig 1.) is the tick vector for Lyme disease in the Northeast and Midwest United States. Larval and nymph stages maintain an efficient horizontal vector ecology mainly with white-footed mice. Along the Pacific coast, the wood rat and Ixodes pacificus make up the predominant enzootic cycle. Asymptomatic chronic infection of other small mammals, birds, and lizards also occurs with B.b and serves to help maintain the organism in nature. Birds play a role as well as phoretic hosts, transporting ticks to different geographic locals without direct infection.
Figure 1: Ixodes scapularis
Ixodes ticks take blood meals as larvae, nymphs, and adults. The entire life cycle requires two years to complete. Ticks acquire and transmit B.b. during both larval and nymph feeding on rodents, who then remain chronically infected, establishing the horizontal enzootic cycle. Humans and other mammals acquire B.b. most commonly from blood meals of nymph ticks. Humans represent accidental, dead end hosts in this complex life cycle.3
Deer are key to winter survival of Ixodes ticks, and serve to provide a blood meal for the adult female Ixodes tick. Deer do not become infected with B.b. due to the innate bactericidal activity of complement proteins in their blood. The female then detaches and lays her eggs in leaf litter on the forest floor. Larvae hatch the following spring.
Epidemiology
Since its discovery in Connecticut in 1977, cases of Lyme disease have increased steadily in the U.S. Changes in the ecology and habitat of the pertinent tick and mammal species, particularly that of deer, have contributed to the distribution and case density. According to CDC case reporting, Lyme disease annual incidence has risen from about 15,000 cases in the mid 1990’s to over 28,000 confirmed cases in 2008. These numbers are an underestimate of the actual caseload by at an estimated 20%.4 In Pennsylvania 3818 confirmed cases were reported in 2008, with an incidence of 30.7 cases per 100,000 population.5
Risk of new infection with Lyme disease begins in April each year, peaks in the summer months, and is minimal after October. The vast majority of cases are identified in June through August each year. Delayed manifestations of Lyme disease can, of course, present year round.
Transmission to Humans
Nymph Ixodes ticks are very small (about 1mm in diameter), and their bite is painless. After attachment and the initiation of the blood meal, changes in spirochete gene expression and translation of new surface proteins (OspA to OspC) must occur before B.b. can be transmitted. The probability of infection in humans after the bite of an infected tick is dependent of the time of attachment and whether the tick is engorged. The risk of infection is 25% with attachment of an engorged tick for > 72 hrs, compared to zero if the observed attachment is < 72 hrs.6 In another study where the duration of attachment was unknown, an un-engorged tick carried an infection risk of only 1%.7 Even in highly endemic areas, only about 15% of Ixodes ticks will harbor B.b.
CLINICAL FEATURES OF LYME DISEASE
The clinical manifestations of Lyme disease can be classified into three main syndromes: early localized disease, early disseminated disease, and late disease. The distinction is to some extent academic, as in clinical practice the stages are not necessarily sequential, and considerable variation in intensity of, and overlap in symptoms may occur. The parallels between the stages and clinical manifestations of Lyme disease and syphilis should be noted.
Early Localized Disease
Erythema migrans (EM), formerly referred to as erythema chronicum migrans, is the most characteristic objective manifestation of Lyme disease (Fig. 2). It appears at the site of the Ixodes tick bite, and a correct diagnosis of EM is pathognomonic for B.b. infection. The lesion is so distinctive that it allowed the description of other associated Lyme disease manifestations years before the discovery of the pathogen, or the development of diagnostic tests specific for B.b..
Figure 2: Erythema migrans

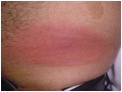
EM appears from a few days to a few weeks after the tick bite in 70-80% of infected individuals. Because the rash is painless, and can occur in skin areas not readily visible, less than 50% of patients in most clinical studies recall the rash. In fact only about 25% of Lyme patients recall a tick bite. EM is a slowly expanding macular erythemetous rash, typically 10-16 cm in diameter (at least 5 cm is required by CDC criteria), that after a few weeks may take on a target-like appearance due to central clearing. The leading edge correlates with the outward migration of spirochetes from the original bite. In about 50% of patients the rash will appear on the thighs, back, or shoulders, but it may escape attention in the axilla, groin, or popliteal fossa. Without specific treatment, the rash may last 2-8 weeks and then will resolve spontaneously. As is the case with syphilis, however, resolution of this first stage lesion does not necessarily indicate resolution of infection.8
Systemic flu-like complaints may accompany the rash. While respiratory symptoms are rare, myalgias, arthralgias, fatigue, headache, fevers, regional adenopathy, and sweats are commonly reported. EM due to B.b. in the United States, as compared with disease in Europe due to other borrelia species, expands more rapidly, is associated with more intense constitutional symptoms and fever, is associated with higher levels of inflammatory cytokines, and is more likely to be associated with spirochetemia.9
Associated lab abnormalities are non-specific and are similar to other summer zoonotic illnesses; they include leukopenia, thrombocytopenia, and hepatocellular enzyme elevations. Significant anemia, however, should suggest co-infection with the malaria-like protozoa Babesia, to be discussed later.10
Early disseminated disease
The similarity to syphilis as a ‘great imitator’ is best demonstrated in the early disseminated phase of Lyme disease. More intense constitutional symptoms may occur within a few days to weeks after the onset of the initial EM lesion, accompanied by multiple skin lesions, which herald early dissemination of B.b., rather than multiple tick bites.
Neurologic manifestations occur after several weeks to months in about 15% of untreated patients, and may include aseptic meningitis, radiculoneuritis, mild cognitive dysfunction, cerebellar ataxia, or cranial neuropathies (esp. Bells palsy). A mild CSF pleocytosis may occur with elevated protein but without hypoglycorrachia.
Lyme carditis most commonly manifests as atrioventricular block. Young otherwise healthy patients may present with pre-syncope and complete heart block. Characteristically, heart block resolves within a few days of appropriate antimicrobial therapy, thus avoiding a costly and unnecessary permanent pacemaker. Myopericarditis is a less commonly encountered manifestation.
Ocular manifestations may involve iritis, choroiditis, or retinal hemorrhage. Panophthalmitis is an ominous yet rare complication. Orchitis, generalized lymphadenopathy, splenomegaly, hepatitis, and hemoproteinuria have also been described.11
Unique manifestations of European Lyme disease, caused by infection with Borrelia afzeli, include Borrelial lymphocytoma, a nodular infiltrative process typically at the dependent portion of the earlobe, along with the dermopathies morphea and acrodermatitis chronica atrophicans.
As is the case for syphilis, many of the early, disseminated features of Lyme disease will abate spontaneously without treatment, and set the stage for subsequent more persistent and potentially devastating late stage complications.
Late Lyme Disease
Late manifestations of Lyme disease occur months to years after initial infection. As a group they are less protean in their presentation but carry greater morbidity. Also, in some cases treatment may produce a less dramatic response and may require more intensive antimicrobial intervention.
Arthritis due to B.b. is best characterized by an oligoarticular large joint inflammatory arthopathy, most commonly presenting as unilateral knee effusion with varying amounts of discomfort. Approximately 60% of untreated Lyme patients will develop some manifestation of joint pain and objective swelling. Over 90% of patients will have knee involvement, with shoulder and or ankle symptoms in about 40%. Small joints and periarticular tendons and bursae may also be involved.12 While less destructive than classical inflammatory arthropathies such as rheumatoid arthritis, degenerative and erosive pathology can occur leading to disability in longstanding cases.
Late neurologic manifestations of B.b. infection range from a mild stable chronic impairment of cognitive function to a progressive dementia. A chronic axonal polyneuropathy presenting as dysaesthesias or radicular pain has also been described. Rare gait disturbances and spastic paraparesis have also been reported. Cerebrospinal fluid from such patients may be non-inflammatory with elevated protein, or show a minimal pleocytosis.13
Lyme Disease and Pregnancy
Based on the serious congenital manifestations associated with maternal syphilis, the possibility of a congenital syndrome related to B.b. spirochete infection was an early concern. However, despite a few initial anecdotal reports of fetal abnormalities, the weight of current evidence does not support the existence of a congenital Lyme syndrome. Five studies now indicate that treated Lyme disease either before or during pregnancy does not carry an increased risk of fetal demise or of congenital anomalies. Furthermore, there is no evidence for transmission of Lyme disease by breastfeeding.
Post-Lyme Syndrome
Several clinical syndromes have bee recognized to occur after completion of treatment for Lyme disease. A case definition for the post-Lyme syndrome has been established by the Infectious Diseases Society of America (IDSA). Characteristic features include waxing and waning of subjective cognitive impairment, diffuse musculoskeletal pain, and chronic fatigue. The diagnosis requires that the patient have had an established diagnosis of Lyme disease by standard criteria, and that a standard antimicrobial treatment course be provided. Objective manifestations of Lyme disease must have been resolved or controlled. The illness must have its onset within 6 months of the diagnosis of Lyme disease, and persist for at least 6 months.14
The term ‘chronic Lyme disease’ is used by some non-Infectious Diseases trained practitioners, as well as patient advocacy groups, to describe a plethora of clinical symptoms above and beyond the post-Lyme syndrome described above. These are alleged to be due to chronic and recalcitrant Lyme infection, but are often without FDA-approved laboratory or objective clinical evidence of true B.b. infection.15 Prolonged courses of antimicrobials are often prescribed, with their own attendant morbidities. The IDSA does not sanction, and in fact warns against, such ill-advised treatment.
STARI
A new syndrome has recently been recognized with very similar features to early Lyme disease. Southern Tick-Associated Rash Infection (STARI, or Masters Disease) is an EM-like illness that occurs in the southern U.S. in areas not endemic for Lyme disease. The tick vector is the Lone Star tick, Amblyoma americanum. While a putative spirochete pathogen (Borrelia lonstarii) has been suggested, STARI patients are seronegative for B.b. No specific diagnostic tests are available. The illness responds to doxycycline and is not known to have long-term sequelae.16
Diagnosis of Lyme Disease
Clinical criteria have been established by the CDC for the diagnosis of Lyme disease.17 The presence of EM is itself diagnostic and constitutes a reportable case. In early, localized disease only 20-40% of patients will be seropositive.10 Serologic testing should be limited to those patients suspected on clinical grounds of infection with B.b. Testing of patients with non-specific symptoms, and therefore a low pre-test probability of Lyme disease, should be discouraged.
In patients beyond the EM stage of illness, with early disseminated and late manifestations described above, laboratory evidence of infection with B.b. is required for the diagnosis, and serologic testing is crucial.
The CDC has established a two-tier conditional strategy for the diagnosis of Lyme borreliosis18. This consists of the use of a sensitive enzyme-linked immunosorbent assay (ELISA) followed by a Western blot immunoassay (WB). The ELISA assay (or in some areas an immunofluorescent assay or IFA) can be associated with a high rate of false positive results, due either to other spirochete infections, or to antibodies to spirochetes present in normal gingival flora. Chronic inflammatory illnesses such as endocarditis, autoimmune diseases, and viral infections can also cause false positive ELISA results. A confirmatory assay is therefore needed to establish a diagnosis of true B.b. infection.
The WB assay detects antibodies to 10 different IgG targets and 3 IgM targets of the Lyme spirochete. The CDC definition of a positive WB is 2 of 3 positive IgM bands, and at least 5 of 10 positive IgG bands. Only positive or equivocal ELISA assays should be subjected to WB confirmation. The strict interpretive criteria requiring at least 5 positive bands must be applied, as up to 50% of normal uninfected persons will show reactivity to at least one WB band. Some laboratories have elected not to apply such strict criteria, resulting in many falsely attributed ‘positive’ results. Unfortunately, many proponents of ‘chronic Lyme disease’ utilize these very labs for their patient testing.
A properly performed ELISA-WB serologic sequence has a high sensitivity and specificity in patients with early or late disseminated disease. Antibodies may persist for years after treatment, however, and therefore serologic testing has no role in proof of cure or in the diagnosis of reinfection.
Other tests are also FDA-approved for the diagnosis of Lyme disease. A one-tier test, the C6 peptide antibody assay, measures antibodies to the VlsE gene products, and has demonstrated sensitivity and specificity similar to the ELISA-WB testing sequence. CSF testing for intrathecal anti-B.b. antibody production has been proposed, but lack of standardization and a high false-negative rate limit its utility.
Polymerase chain reaction (PCR) testing for B.b. DNA in synovial fluid or CSF is also available, but suffers from both false-positive and false-negative performance issues. PCR is not FDA-approved for, and should not be used in the testing of, blood or urine. Similarly, the marketed Lyme urine antigen test is of no proven value, and is not recommended for diagnostic use. Other diagnostic modalities have been introduced, such as Immune Complex Disruption assays, T-cell proliferative response assays, and B-cell chemo-attractant assays. None of these have been validated sufficiently for clinical use, and for now should be avoided.
Finally, cultivation of B.b. has been accomplished on very specialized media, but the techniques are not available outside of specialized research facilities.
Treatment of Lyme Disease
Antimicrobial treatment of Lyme disease varies by stage of disease and clinical manifestations. The IDSA has published definitive guidelines in comprehensive Tables.14 A general overview will be presented here.
In considering the various regimens for treatment, relative differences in efficacy and phamacokinetics must be considered. However, in the non-allergic, non-pregnant adult patient, the oral regimen of choice is doxycycline 200-400 mg daily in Q12 hour divided doses. For parenteral regimens, ceftriaxone at a dose of 2gms IV daily is preferred.
EM, or early, localized Lyme disease, can be treated in adults with an oral antibiotic regimen of amoxicillin or doxycycline for two weeks. There is no advantage to IV antimicrobials for this stage of illness, as the risk of development of later stages is negligible. The rash and somatic complaints may last a few weeks, but if treated at this stage essentially all patients will be asymptomatic at 3 months follow up.
Management of early neurologic manifestations of Lyme disease depends on the specific manifestations. For patients with isolated Bell’s palsy, with no clinical evidence of meningoencephalitis or radiculopathy, treatment can consist of an oral doxycycline regimen. In patients with evidence of aseptic meningitis or encephalitis, a parenteral regimen with ceftriaxone is preferred. The performance of a lumbar puncture in the setting of Bell’s palsy is controversial. The CSF may demonstrate a pleocytosis even without clinical signs of meningoencephalitis. Furthermore, for patients with Bell’s palsy the response to IV ceftriaxone is not superior to that of oral doxycycline, and other neurologic sequelae are rare with either treatment approach.
Cardiac Lyme disease is treated in the vast majority of cases with parenteral antibiotics. There is at least the theoretical concern of progression of first degree AV block with initiation of antimicrobials. In patients hospitalized with symptomatic advanced AV nodal block, initiation of parenteral ceftriaxone with monitoring by cardiac telemetry is appropriate. Treatment for 14-21 days is indicated for most patients.
Late Lyme disease outside of the central nervous system can usually be managed with oral antimicrobials. Arthritis without neurologic disease should be treated with a 28-day course of oral doxycycline as the preferred regimen. It should be noted that the clinical response in late stage patients is often frustratingly slow, but that continued clinical benefit accrues in the weeks after treatment has been completed. For patients with recurrent disease after an oral regimen, a repeat of the same regimen or a parenteral course of ceftriaxone can be considered.
For late Lyme encephalitis or radiculitis, a 28-day course of ceftriaxone is indicated. As is the case with tertiary syphilis in the CNS, serial lumbar punctures may be needed to monitor the laboratory and clinical responses to treatment.
Four randomized, placebo-controlled trials have now conclusively demonstrated that antimicrobial intervention provides no benefit to patients with Post-Lyme syndrome. Exclusion of alternative diagnoses and symptomatic therapy is the preferred approach.
The proper treatment of Lyme disease remained controversial. In 2008, after intense lobbying by Lyme advocacy groups, the Connecticut Attorney General ordered a re-evaluation of the 2006 IDSA treatment guidelines. A special independent Review Panel was convened. In April 2010, after a two year review, the panel unanimously upheld the 2006 guidelines.
Prevention
Prevention of tick bites is of course the best way to avoid Lyme disease. Avoidance of tick-infested areas is optimal. As this is often not feasible, daily inspection for attached ticks is key, remembering that ticks must be attached for 48 hours to effectively transmit B.b.. Light-colored clothing with long sleeves, and long pants tucked into one’s socks, help identify unattached ticks. The use of chemical sprays containing permethrin (for clothes) and DEET (for skin) provide some protection as well. Attached ticks should be removed with forceps by grasping the tick at the skin attachment site, and pulling straight out. Care must be taken not to put pressure on, or injure the body of the tick, as this may result in ‘self inoculation.’ The site should be disinfected and observed for the development of EM for 30 days.
Post-bite prophylaxis with doxycycline 200 mg has been shown to be efficacious in reducing the risk of Lyme disease in highly endemic areas if given within 72 hours after the bite of an engorged Ixodes tick.7 The cost effectiveness has been challenged, however, and simple expectant observation is also an appropriate option.14 Serologic testing for a tick bite is not indicated.
A vaccine against B.b. (Lymerix) was introduced in 1998. This unique vaccine was based on a recombinant OspA molecule. The mechanism of action was to generate anti-OspA antibodies, which upon the initiation of the blood meal would enter the tick gut and kill B.b. before the expression of the OspC could occur. The vaccine efficacy was proven, at least in the short term. The vaccine was withdrawn from the market over concerns about the possible cross-reaction of vaccine-induced antibodies with host synovial proteins, possibly leading to chronic autoimmune synovitis.
Coinfection
A unique feature of Ixodes ticks is the ability to transmit multiple pathogens simultaneously. Infection with Lyme disease in the northeast United States in spring and summer months also carries the risk of infection with two additional important pathogens: Anaplasmosis and Babesiosis.
HUMAN GRANULOCYTOTROPIC ANAPLASMOSIS (HGA)
Microbiology
The agent of HGA (formerly Human Granulocytic Ehrlichiosis) is Anaplasma phagocytophilum. (Formerly Ehrlichia phagocytophila, and Ehrlichia equi). These are small, obligate intracellular Gram-negative bacilli. They have a tropism for neutrophils, in which they grow in a host cell vacuole as a micro-colony called a morula (Fig. 3).
Figure 3: Morula of Anaplasma phagocytophilum in a host granulocyte
Epidemiology
Ixodes scapularis and I. pacificus, the same vectors seen in Lyme disease, also transmit HGA. The small mammal and deer ecology is also similar, as is the geographic distribution of disease. Over 4,000 cases of HGA have been identified in the United States since 1999, despite the fact that it is not a reportable disease in most states. Small numbers of cases have recently been reported in Europe as well.
Clinical Features
After an incubation period of 1-2 weeks, HGA presents as an acute febrile illness with headache, myalgias, arthralgias, and malaise. Nausea, vomiting, stiff neck, and confusion may occur. There is no characteristic rash, although EM can be seen in cases of coinfection. In severe cases, respiratory failure, septic shock, renal failure, and rhabdomyolysis can occur. Meningoencephalitis resulting in coma can occur as the illness advances. Late stages are characterized by complicating opportunistic infections.19 Similarities in presentation and severity have led to this disease being referred to as ‘spotless Rocky Mountain Spotted Fever’.
Laboratory Features
HGA is characterized by the triad of leukopenia (neutropenia with a marked left shift), thrombocytopenia, transamminitis, and azotemia. The CSF may demonstrate pleocytosis. Bacteremia is detectable only by special techniques, and can last for 28 days in untreated patients.
Diagnosis
Both of the complicating coinfections that may present in association with Lyme disease are characterized by the importance of the peripheral blood smear in their diagnosis. Morulae (Fig. 3) are identified in buffy coat peripheral blood neutrophils in 20-80% of patients with HGA. The organism is difficult to culture, but the diagnosis may be confirmed utilizing PCR amplification. This technique has been shown to have a sensitivity of 54-86%, and a specificity near 100%. Serologic testing of acute and convalescent sera is also available, but is hampered by a lack of sensitivity early in infection, as well as a high baseline rate of seropositivity in endemic areas.19
Treatment
Mild cases may resolve without treatment in about 10 days. Treatment with doxycycline or tetracycline is highly effective, producing rapid defervescence in 48 hours from the initiation of treatment. Unlike Rocky Mountain spotted fever, however, chloramphenicol is ineffective in HGA. Despite the prolonged bacteremia described above, the overall case fatality rate is less than 5%, and true chronic infection does not occur.
BABESIOSIS
Microbiology
The causative agents of human Babesiosis in the U.S. are Babesia microti (East coast) and B. duncani (west coast). These are obligate intra-erythrocytic protozoan parasites. Babesia is classified in the same phylum as Plasmodium, Toxoplasma, and Cryptosporidium species. The life cycle involves sexual reproduction in arthropods, and asexual reproduction in mammals.20
Epidemiology
The expansion of the habitat of the white-tailed deer in the U.S. has contributed to the establishment of a well-defined endemic zone from southern New England and New York to New Jersey, as well as the central Midwest. All three of the life cycle stages of Ixodes scapularis are capable of transmitting disease. The incidence of Babesiosis is rising faster that that for Lyme disease in endemic areas. While tick bites represent the predominant mode of transmission to humans, blood transfusion and transplacental transmission also occur.
Clinical Features
Illness begins 1-6 weeks after the bite of an Ixodes tick. The clinical spectrum of presentations ranges from asymptomatic infection, to a self-limited summertime febrile illness, to a fulminant relapsing or even fatal course. Patients at increased risk for fulminant disease include those with prior splenectomy, HIV infection, malignancy, or immunosuppressive illness.
Patients most commonly present with fever, chills, arthralgias, myalgias, and cough. Abdominal pain, vomiting, conjunctival hemorrhages, headache, and photophobia may ensue. Examination may reveal only fever and mild hepatosplenomegaly. The illness may last for weeks to months in a relapsing and remitting pattern. Mortality overall is less than 5%, although for patients who are hospitalized it exceeds 10%.
Diagnosis
Hemolytic anemia is rarely seen initially. Low-level parasitemia may, in fact, escape detection. The formation of diagnostic tetrads of merozoites (Fig. 4), the so-called Maltese cross, is in fact rare. PCR amplification is available and can aid in the diagnosis. IFA or EIA serology can also be utilized, as can hamster inoculation.
Figure 4: Babesia species demonstrating merozoites in tetrad or ‘Maltese Cross’
Treatment
In asymptomatic patients discovered incidentally, no treatment is needed unless parasitemia lasts longer than 3 months. Patients who recover from symptomatic disease but are smear and PCR-negative do not require treatment. Symptomatic patients with evidence of ongoing parasitemia should be treated.
For mild to moderate illness, treatment should consist of combined azithromycin and atovaquone for 7-10 days. Peripheral smears are then followed for 3 months for evidence of relapse or persistence. A positive PCR after treatment may detect DNA from killed parasites, and therefore is not indicative of treatment failure. Patients with severe Babesiosis associated with multisystem organ failure are treated with IV clindamycin and enteral quinine. Intravenous pentamidine and trimethoprim-sulfa can also be utilized. Finally, exchange transfusion can be successful in the most profoundly ill patients with parasitemia levels exceeding 10%. Even after recovery, however, chronic low-grade infection in survivors may persist for years, and resist eradication.20
SUMMARY
In the Northeastern United States, Ixodes ticks can transmit three pathogens simultaneously: Lyme disease, Human Granulocytic Anaplasmosis, and Babesiosis, thus confronting the clinician with a baffling mix of clinical signs, symptoms, and laboratory clues. As summer is upon us, we must all be cognizant of these important infections in our approach to febrile patients.
REFERENCES
1 Steere AC, et al. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum 1977;20(1):7-17.
2 Burgdorfer W, et al. Lyme Disease- a tick-borne spirochetosis? Science 1982;216:1317-9
3 Tilly K, et al. Biology of Infection with Borrelia burdorferi. Infect Dis Clin N Am 2008;22:217-34
4 www.cdc.gov/ncidod/dvbid/lyme/ld_UpClimbLymeDis.htm
5 www.cdc.gov/ncidod/dvbid/lyme/ld_rptdLymeCasesbyState.htm
6 Nadelman RB, et al. Prophylaxis with single-dose doxycycline for the prevention of Lyme disease after an Ixodes scapularis tick bite. N Engl J Med 2001; 345(22):79-84
7 Shapiro ED, et al. A controlled trial of antimicrobial prophylaxis for Lyme disease after deer-tick bites. N Engl J Med 1992; 327(25):1769-73
8 Nadelman RB, et al. Erythema migrans and early Lyme disease. Am J Med 1995;98(4A):15S-23S
9 Jones KL, et al. Higher mRNA levels of chemokines and cytokines associated with macrophage activation in erythema migrans skin lesions in patients from the United States than in patients from Austria witn Lyme borreliosis. Clin Infect Dis 2008;46:85
10 Dandache P, et al. Erythema Migrans. Infect Dis Clin N Am 2008 (22):235-60
11 Steere AC, Lyme disease. N Engl J Med. 1989;321:586
12 Puius YA and Kalish RA. Lyme arthritis: Pathogenesis, clinical presentation, and management. Infect Dis Clin N Am 2008;22:289-300
13 Halperin JJ. Nervous System Lyme Disease. Infect Dis Clin N Am 2008;22:261-74
14 Wormser GP. The clinical assessment, treatment, and Prevention of Lyme disease, Human Granulocytic Anaplasmosis, and Babesiosis: Clinical Practice Guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43(9):1089-1134.
15 Feder HM et al. A critical appraisal of “chronic Lyme disease” N Engl J Med 2007;357:1422
16 Masters EJ. Et al. STARI, or Masters Disease: Lone Star Tick-Vectored Lyme-Like Illness. Infect Dis Clin N Amer 2008;22:361-76
17 Centers for Disease Control and Prevention. Case definitions for infectious conditions under public health surveillance. MMWR Morb Mortal Recomm Rep 1997;46(RR-10);1
18 Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep 1995;44:590.
19 Bakken, JS and Dumler, S. Human Granulocytic Anaplasmosis. Infect Dis Clin N Am 2008;22:433-48.
20 Vannier, E, et al. Human Babesiosis. Infect Dis Cin N Am 2008;22:469-88.