 Click to Print Adobe PDF
Click to Print Adobe PDF
Summer 2010 - Vol.5, No.2
Peri-Operative Autotransfusion: A Vital Component of Blood Management
Craig J. Gassmann, CCP
Patricia R. Carl, CMA
|
 |
INTRODUCTION
In order to effectively manage the nation’s blood supply, modification of blood utilization practices has become a high priority, but that objective has been made especially challenging by society’s evolving demographics. The population of the United States is aging,1 and the first wave of Baby Boomers will reach the full retirement age of 65 in 2011. From then on, 10,000 new retirees will be added to Social Security and Medicare every day for 20 years.1 One unrecognized consequence of this demographic shift is the impact on our blood supply. According to the American Association of Blood Banks (AABB), the cutoff age for potential blood donors is 60 years of age. Therefore, as our population ages, the number of potential blood donors will decrease, while the number of potential blood recipients will increase. A severe shortage of blood and blood components may develop in the foreseeable future, unless it is offset by a significantly increased supply, or by reduced usage of blood and blood components.2 3
To offset this concern, a systematic approach to Blood Management has evolved with an emphasis on quality, safety and cost efficiency of blood component therapy. The cornerstones of blood management programs are the implementation of evidence-based transfusion guidelines to reduce variability in transfusion practice, and the employment of multidisciplinary teams to study, implement, and monitor local blood management strategies. At Lancaster General Hospital, the Blood Utilization Review Committee has established evidence-based transfusion guidelines, and functions as the multidisciplinary team that monitors hospital blood management strategies. One of those strategies is Peri-Operative Autotransfusion, or the collection, processing, and reinfusion of the patient's own blood that is lost during the peri-operative period.
Autotransfusion is uniquely advantageous because it directly reduces the demand for banked blood, while it simultaneously eliminates the risks of allogeneic blood transfusions.
CONSEQUENCES OF TRANSFUSION
In spite of the fact that the U.S. blood supply is the safest it has ever been, allogeneic blood transfusion is still associated with significant risks. The public mistakenly believes that most of the risk of allogeneic transfusion involves transmission of human immunodeficiency virus (HIV), but in fact, the most significant risks in 2006 were unrelated to viral transmission.4 Improved donor screening and testing of donated units has decreased the risk of hepatitis and HIV to less than 1 of every 1,000,000 transfusions.4 Mistransfusion - administration of blood products to the wrong patient - is now one of the leading causes of transfusion complications. In spite of increased awareness and vigilance, mistransfusion still occurs in approximately 1 of every 14,000 units transfused.5 Death occurs in this group in 1 of 600,000 – 800,000 transfusions.6
Worldwide, the leading cause of transfusion related morbidity and mortality is Transfusion Related Acute Lung Injury, (TRALI), with an estimated frequency of 1 of 500 platelet transfusions and 1 of 1,000 – 5,000 plasma and red blood cell transfusions.7-11 It is likely that the actual incidence of TRALI is higher than reported, due to a lack of awareness of the syndrome on the part of many clinicians.
PATHOPHYSIOLOGY OF TRANSFUSION-RELATED INJURY
TRALI and the Systemic Inflammatory Response Syndrome, (SIRS) are related to the buildup of mediators of inflammation in stored blood. Cytokines released from the residual leukocytes appear to be the primary concern, although complement activation has been implicated also.12 In addition, hemolysis of RBCs releases intracellular contents and raises serum potassium levels. This “storage lesion” is cumulative and worsens with prolonged storage. Several studies have associated “older” blood with higher morbidity and mortality.12-14Currently, the AABB allows RBCs to be stored for up to 42 days.
TRALI can also be caused by the presence in donor plasma of antibodies to human leukocyte antigen. These antibodies, which attack recipient white blood cells, are most prevalent in plasma products donated by multiparous females. In fact, the UK has recently converted to male-only plasma donations, and the US is likely to follow this lead.15
Prolonged storage also impairs the ability of stored RBCs to deliver oxygen to the tissues.16,17 2,3 diphosphoglyceric acid, (2,3,DPG), a substance produced in RBCs to aid in transfer of oxygen to tissues, is depleted quickly in stored blood and it can take 24 to 48 hours to replenish 2,3,DPG levels. Until 2,3,DPG levels are restored, transfused red cells have very limited participation in tissue oxygenation.
Another under-recognized complication of allogeneic transfusion is Transfusion Related Immunomodulation (TRIM). TRIM-invoked immunologic changes include both stimulation of humoral immunity resulting in production of allo-antibodies, and down-regulation of cellular immunity resulting in altered host defenses.18-20 Since transfusions generally only occur in patients already stressed by surgery or illness, TRIM is thought to contribute to the consistent finding of stepwise increases in infection rates21-26, ventilator support times25, ICU and hospital length of stays.27-29 and short term and long term mortality in patients who receive transfusions.14.26-29 Also, several studies have shown an increase in cancer recurrence rates in transfused vs. non-transfused patients.30-32
CRITERIA FOR TRANSFUSION
Despite mounting evidence that unnecessary transfusions can cause serious harm, several studies have documented a lack of compliance with appropriate transfusion guidelines, as well as tremendous variations in transfusion practices among different institutions and even among individual physicians within the same institution.33-37
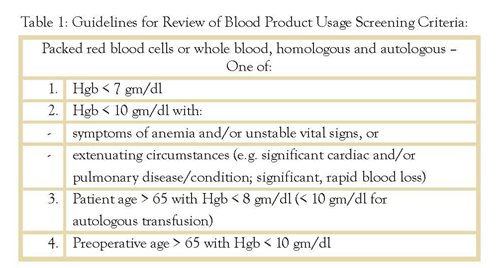
Lancaster General Hospital’s Blood Utilization Review Committee has established Blood Product Usage Screening Criteria based on the simple and unexceptionable principle that the benefit of a transfusion should outweigh its risk. To that end, the criteria state that patients should not receive donor banked blood unless the hemoglobin level is less than 7, except with extenuating circumstances. (Table 1)
COST OF ALLOGENEIC TRANSFUSIONS
The “true” cost of an RBC transfusion is difficult to estimate. Aside from the “direct” costs of acquiring, cross-matching, testing and infusing the blood, there are also “indirect” costs, such as the time physicians spend explaining the risks of transfusion and getting informed consent, or the time nurses spend monitoring the transfusion. There is also the time spent transporting blood around the hospital and cleaning up the waste, and the overhead cost of storing and controlling the blood in the blood bank and discarding any unused blood. Shander’s group at the The New Jersey Institute for Bloodless Medicine and Surgery, Englewood Hospital and Medical Center, Englewood, NJ, attempted to measure all these direct, indirect and overhead costs at their institution, and found the total cost of transfusing a unit of RBC in 2007 was $1,158.38 This total was the cost to the institution, not the patient charge, and it consisted of: indirect overhead cost- 40.6%, transfusion processing cost -34.0%, weighted average acquisition cost - 21.5%, and direct overhead cost - 3.9%.
Shander’s analysis did not include the cost of treating any adverse events related to allogeneic transfusions. (There were 21 mild or moderate reactions in the 461 patients who received transfusions.) Nonetheless, the cost of treating even a minor transfusion reaction adds significantly to the indirect costs of allogeneic transfusions, not to mention the cost of treating any infection that results from immuno-modulation. Several studies have shown that patients who receive no transfusions, or autotransfusion RBC’s alone, have shorter LOS, and lower overall costs.38-40
PERIOPERATIVE AUTOLOGOUS BLOOD MANAGEMENT AND AUTOTRANSFUSION
Surgical procedures account for a high percentage of blood transfusions,41 and anything that can be done to reduce blood utilization during and after surgery will have a substantial impact on transfusion requirements. Autotransfusion is one of the most important such interventions.
Autotransfusion involves the collection, washing, and reinfusion of blood shed at the surgical site. Intraoperatively, suction is used to collect shed blood in a controlled manner to a dedicated device rather than to a discard circuit. Heparin or Anticoagulant Citrate Dextrose is infused into the shed blood to keep it from clotting. Once enough blood has accumulated, it is centrifuged in the autotransfusion device to separate the RBCs from the waste products, and the RBCs are washed with 0.9% NaCl to remove any unwanted contaminants. Finally, the washed RBCs are filtered and reinfused to the patient. Since the patients receive their own fresh RBCs, the cells have high levels of 2,3DPG, and will be immediately active in tissue oxygenation. The RBC’s never leave the surgical area, are never put into the banked blood pool, do not incur storage expense, and have virtually no chance of a clerical error or mistransfusion. Moreover, problems with undesirable immune responses are mitigated.
Autotransfusion is contraindicated in some cancer operations, any operation with bacterial contamination, and in sickle cell disease. Otherwise, any operation in which there is the possibility of significant blood loss has the potential for autotransfusion. Intraoperative autotransfusion is performed in cardiac, vascular, orthopedic, neurosurgical, gynecological, and general surgery procedures, as well as during the management of trauma.
A major application of postoperative autotransfusion is in orthopedic surgery, primarily in joint replacement surgery. Drains are placed in the surgical site and blood shed postoperatively is collected and anticoagulated with Anticoagulant Citrate Dextrose. From that point the process is similar to intraoperative autotransfusion, with centrifugation, washing, filtering and reinfusion.
AUTOTRANSFUSION AT LANCASTER GENERAL HOSPITAL
Following are data about the number of patients and the autotransfusion volumes of RBCs reinfused over the last three calendar years (2007-2009) at Lancaster General Hospital:
Volumes: Intraoperative Reinfusion Volume: 1,795,504 cc's Postoperative Reinfusion Volume: 872,763 cc's Total: 2,668,267 cc's
 A “unit” of allogeneic banked RBCs is approximately 250 cc. The total volume of reinfused RBCs is the equivalent of: 10,672 “units” of RBCs. This is an average of 425 cc’s per patient.
A “unit” of allogeneic banked RBCs is approximately 250 cc. The total volume of reinfused RBCs is the equivalent of: 10,672 “units” of RBCs. This is an average of 425 cc’s per patient.
 CONCLUSION
Peri-operative Autotransfusion is an essential part of a comprehensive Blood Management program. It is a safe and cost effective technique to reduce the strain on the blood bank, and to ensure patients get their own blood back. It has been used effectively at Lancaster General Hospital, salvaging a massive amount of blood that would have otherwise been lost, and returning it to its rightful owner, the patient.
CONCLUSION
Peri-operative Autotransfusion is an essential part of a comprehensive Blood Management program. It is a safe and cost effective technique to reduce the strain on the blood bank, and to ensure patients get their own blood back. It has been used effectively at Lancaster General Hospital, salvaging a massive amount of blood that would have otherwise been lost, and returning it to its rightful owner, the patient.
REFERENCES
1 http://www.aoa.gov/AoARoot/Aging_Statistics/future_growth/future_growth.aspx
2 Zou, S. et al. Changing age distribution of the blood donor population in the United States. Transfusion. 2008; 48(2);251-7
3 Crawford, SQ. et al. Regional and temporal variation in American Red Cross blood donations, 1995 to 2005. Transfusion. 2008; 48(8):1576-83
4 Goodnough LT. Risks of Blood Transfusions. Crit Care Med 2003;31:S67886
5 Goodnough LT, et al. Transfusion medicine: Looking to the future. Lancet, 2003;361(9352): 161-169.
6 Linden JV. et al. Transfusion Errors in New York State: an analysis of 10 years experience. Transfusion. 2000;40:207-13
7 Silliman CC. Transfusion-related acute lung injury. Blood 2005;105:2266-73–
8 Fransen E. et al. Impact of blood transfusions on inflammatory mediator release in patients undergoing cardiac surgery. Chest 1999;116:1233-9
9 Toy P, et al. Transfusion-related acute lung injury: definition and review. Crit Care Med 2005;33:721-6–
10 Boshkov LK. Transfusion-related acute lung injury and the ICU. Crit Care Clin 2005;21:479-95.
11 Popovsky MA. Transfusion-related acute lung injury. Transfusion 1995;35:180-1.
12 Kristiansson M, et al. Cytokines in stored red blood cell concentrates: promoters of systemic inflammation and simulators of acute transfusion reactions? Acta Anaesthesiol Scand 1996;40:496-501.
13 Tinmouth A, et al. Clinical consequences of red cell storage in the critically ill. Transfusion 2006;46:2014-27.
14 Gorman Koch, C. et al. Duration of red-cell storage and complications after cardiac surgery. 2008; N Engl J Med;358;12 1229-39
15 Eder AF, et al. Transfusion-related acute lung injury surveillance (2003–2005) and the potential impact of the selective use of plasma from male donors in the American Red Cross. Transfusion 2007;47:599-607.
16 Suttner S, et al. The influence of allogeneic red blood cell transfusion compared with 100% oxygen ventilation on systemic oxygen transport and skeletal muscle oxygen tension after cardiac surgery. Anesth Analg 2004;99:2-11.
17 Tsai AG, et al. Microvascular perfusion upon exchange transfusion with stored red blood cells in normovolemic anemic conditions. Transfusion 2004;44: 1626-34.
18 Blumberg N. Deleterious clinical effects of transfusion immunomodulation: proven beyond a reasonable doubt. Transfusion 2005;45:S33-9.
19 Landers DF, Hill GE, Wong KC, Fox IJ. Blood transfusion-induced immunomodulation. Anesth Analg 1996;82:187-204.
20 Blumberg N, Heal JM. Immunomodulation by blood transfusion: an evolving scientific and clinical challenge. Am J Med 1996;101:299-308.
21 Carson JL, Altman DG, Duff A, et al. Risk of bacterial infection associated with allogeneic blood transfusion among patients undergoing hip fracture repair. Transfusion 1999;39:694-700.
22 Leal-Noval SR, Rincon-Ferrari MD, Garcia-Curiel A, et al. Transfusion of blood components and postoperative infection in patients undergoing cardiac surgery. Chest 2001;119: 1461-8.
23 Claridge JA, Sawyer RG, Schulman AM, et al. Blood transfusions correlate with infections in trauma patients in a dose-dependent manner. Am Surg 2002;68:566-72.
24 Taylor RW, Manganaro L, O'Brien J, et al. Impact of allogenic packed red blood cell transfusion on nosocomial infection rates in the critically ill patient. Crit Care Med 2002;30:2249-54.
25 Shorr AF, Duh MS, Kelly KM, Kollef MH. Red blood cell transfusion and ventilator-associated pneumonia: a potential link? Crit Care Med 2004;32:666-74.
26 Hill GE, Frawley WH, Griffith KE, et al. Allogeneic blood transfusion increases the risk of postoperative bacterial infection: a meta-analysis. J Trauma 2003;54:908-14.
27 Moore FA, Moore EE, Sauaia A. Blood transfusion: an independent risk factor for postinjury multiple organ failure. Arch Surg 1997;132:620-4.
28 Shapiro MJ, Gettinger A, Corwin HL, et al. Anemia and blood transfusion in trauma patients admitted to the intensive care unit. J Trauma 2003;55:269-73.
29 Malone DL, Dunne J, Tracy JK, et al. Blood transfusion, independent of shock severity, is associated with worse outcome in trauma. J Trauma 2003;54:898-905.
30 Langley SM, Alexiou C, Bailey DH, Weeden DF. The influence of perioperative blood transfusion on survival after esophageal resection for carcinoma. Ann Thorac Surg 2002;73:1704-9.
31 Vamvakas EC. Transfusion-associated cancer recurrence and postoperative infection: meta-analysis of randomized, controlled clinical trials. Transfusion 1996;36:175-86.
32 Blumberg N, Heal JM. Effects of transfusion on immune function: cancer recurrence and infection. Arch Pathol Lab Med 1994;118:371-9.
33 Corwin HL, et al. The CRIT study: anemia and blood transfusion in the critically ill; current clinical practice in the United States. Crit Care Med 2004;32:39–52.
34 Goodnough LT. et al, for the Transfusion Medicine Academic Award Group. The variability of transfusion practice in coronary artery bypass surgery. JAMA 1991;265:86–90.
35 Stover EP, et al, for the Institutions of the MultiCenter Study of Perioperative Ischemia Research Group. Institutional variability in red blood cell conservation practices for coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth 2000;14:171–6.
36 Audet AM, et al. Red blood cell transfusion practices in patients undergoing orthopedic surgery: a multi-institutional analysis. Orthopedics 1998;21:851–8.
37 Poses RM. et al. How you look determines what you find: severity of illness and variation in blood transfusion for hip fracture. Am J Med 1998;105: 198–206.
38 Shander, Aryeh. Et al. The true cost of red blood cell transfusions in surgical patients. 2008: Presented at the 50th Annual American Society of Hematology Meeting, The Moscone Center Poster Board III-127
39 Sonnenberg FA, et al. The cost-effectiveness of autologous transfusion revisited: implications of an increased risk of bacterial infection with allogeneic transfusion. Transfusion 1999;39:808–17.
40 Blumberg N, et al. A cost analysis of autologous and allogeneic transfusions in hip-replacement surgery. Am J Surg 1996;171:324–30.
41 Anderson, SA. et al. Blood use by inpatient elderly population in the United States. Transfusion, 2007(4):582-92