 Click to Print Adobe PDF
Click to Print Adobe PDF
Summer 2010 - Vol.5, No.2
Endoscopic Ultrasound of the Gastrointestinal Tract
Ketan Kulkarni, M.D.
Regional Gastroenterology Associates of Lancaster
|
 |
Abstract
Endoscopic ultrasound (EUS) combines endoscopy with the ability to perform ultrasonography within the gastrointestinal lumen. Detailed images are obtained of the gastrointestinal wall layers as well as structures and organs close to the gastrointestinal tract. Fine needle aspiration (FNA) allows sampling of nearby suspicious masses and lymph nodes. EUS is the most accurate imaging modality available for the local and regional staging of esophageal, gastric and rectal cancer; it has a central role in the diagnosis and staging of pancreatic cancer; and it is being more commonly utilized for the staging of non-small cell lung cancer. It facilitates identification of patients who may have a resectable malignancy, and those who are candidates for neoadjuvant therapy. Given its ability to visualize the gastrointestinal wall layers as well as the pancreatico-biliary system, EUS is ideally suited for investigating a variety of benign conditions, such as gallstone disease and non-mucosal lesions in the gastrointestinal tract. The EUS program at Lancaster General Hospital has demonstrated steady growth and has already had a significant clinical impact.
Introduction
Endoscopic ultrasound (EUS) has become an integral part of the management of a variety of gastrointestinal conditions. The coupling of ultrasonography and endoscopy has allowed physicians to better visualize and stage many cancers within or adjacent to the gastrointestinal tract. Currently, EUS has a pivotal role in the local and regional staging of esophageal, non-small cell lung, gastric, pancreatic, biliary and rectal cancer. As technology continues to advance, EUS is being utilized more commonly in benign conditions as well. This article will discuss the various indications for EUS and, more importantly, how endoscopic ultrasound can change the clinical management of patients. The experience with endoscopic ultrasound since it was introduced at Lancaster General Hospital in August of 2009 will also be described.
The Endoscopic Ultrasound Procedure
Endoscopy of the upper gastrointestinal tract is accomplished by introducing an endoscope into the oropharynx and advancing it to the duodenum. Similarly, colonoscopy can be utilized to investigate the lumen of the colon, most commonly for purposes of screening for colon cancer. An EUS scope is a specially designed echoendoscope with an ultrasonographic transducer at its tip which allows one to view ultrasonographic images from within the lumen of the GI tract. Since the EUS scope is within the lumen, and directly adjacent to the gastrointestinal wall, it obtains detailed images of the GI tract which are especially useful in determining the depth of invasion of a variety cancers, and assessing adjacent organs such as the pancreas. Furthermore, a fine needle aspiration (FNA) needle can be introduced through the echoendoscope to sample masses or enlarged lymph nodes
Within the mediastinum, endoscopic ultrasound is critically important in the staging of esophageal and non-small cell lung cancer, as well as for the evaluation of masses, lymph nodes and cysts located within the posterior mediastinum. In esophageal cancer, multiple studies have clearly shown that EUS is superior to a CT scan for T staging (depth of tumor invasion) as well as N staging (lymph node staging).1 Endoscopic ultrasound is particularly important when trying to decide which patients will benefit from preoperative neoadjuvant therapy, and which patients can proceed directly to surgery. In fact, studies have demonstrated that endoscopic ultrasound is associated with an overall survival advantage in esophageal cancer patients, most likely through appropriate administration of neoadjuvant therapy due to more accurate preoperative staging.2 While EUS is the best modality available for local and regional staging, CT and PET scans are superior at determining metastatic disease in the liver or lung. Therefore, these modalities should be thought of as complementary studies when staging a patient with esophageal cancer. While EUS provides excellent views of the gastrointestinal wall, its utility is limited for restaging after neoadjuvant therapy where it has not been shown to be accurate. This experience is likely due to the fact that the inflammation and fibrosis associated with radiation and chemotherapy are indistinguishable from residual tumor. Nonetheless, EUS can play a role in sampling residual lymph nodes after neoadjuvant therapy to confirm persistent nodal metastases.
Because of its accuracy in assessing mediastinal lymph nodes, endoscopic ultrasound also has a growing role in the pre-operative management of patients with non-small cell lung cancer. We know that patients with mediastinal lymph node metastasis (stage III) are preferably treated with multimodal therapy, whereas patients with less advanced disease should proceed directly to surgery.3 As many as one third of patients with lung cancer present with mediastinal lymph node metastases. Furthermore, while PET CT can detect nodal disease in the mediastinum, the false positive rate for PET scans is as high as 28% and thus tissue is still needed to confirm nodal involvement.4
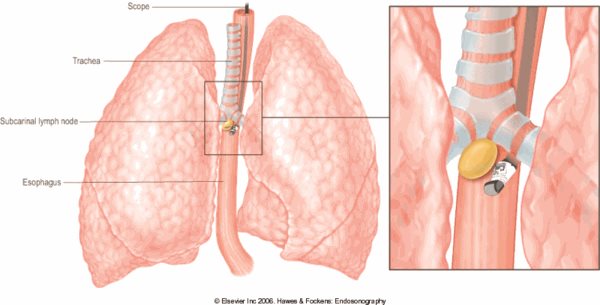
To evaluate and sample mediastinal lymph nodes, mediastinoscopy was traditionally needed, but EUS provides a much less invasive and less risky technique for sampling lymph nodes in the posterior mediastinum, subcarinal space, and aorto-pulmonary window (Figure 1). And though the air-filled trachea does not allow EUS to evaluate the entire mediastinum, endobronchial ultrasound (recently introduced at Lancaster General Hospital), can evaluate the remainder of the mediastinum. The combination of endoscopic ultrasound and endobronchial ultrasound has been shown to have a sensitivity of 93% and a negative predictive value of 98% to 99% for detection of mediastinal lymph node involvement in patients with non-small cell lung cancer5 Studies have clearly shown that EUS can prevent unnecessary mediastinoscopies and thoracotomies3 In addition, EUS can be utilized to sample suspicious lymph nodes after neoadjuvant therapy in lung cancer patients to see if they have been downstaged by chemotherapy and would now benefit from surgery.
Figure 1: Diagram showing an EUS-guided FNA of a subcarinal lymph node. EUS provides access to lymph nodes in the subcarinal space, aortopulmonary window, paraesophageal space, pulmonary ligamentum and the lower paratracheal space.
Pancreas and Biliary System
The location of the pancreas close to the stomach and duodenum allows for optimal visualization of the entire gland by EUS (Figure 2). In fact pancreatic evaluation was one of the first indications for endoscopic ultrasound. In addition to the staging of pancreatic cancer, EUS is also important in diagnosing cholelithiasis, choledocholithiasis, chronic pancreatitis and pancreatic cysts.
Figure 2: Illustration depicting the location of the pancreas in relation to the stomach and duodenum. This illustration clearly shows why endoscopic ultrasound provides detailed images of the entire pancreas.
 The differential diagnosis of pancreatic cysts is becoming more common with the ever-increasing reliance upon radiologic imaging for the evaluation of patients. Numerous types of cysts can occur in the pancreas, including serous cystic neoplasms, mucinous cystic neoplasms (MCN), intraductal papillary mucinous tumors (IPMT), and pseudocysts. Prior studies have documented the considerable malignant potential of MCNs and IPMTs.6 Therefore, accurate diagnosis of pancreatic cysts is of paramount importance. Endoscopic ultrasound allows the operator to aspirate fluid from pancreatic cysts for cytology as well as amylase and CEA levels to help distinguish mucinous cysts from cysts with no malignant potential. The large Cooperative Pancreatic Cyst Study demonstrated that a cyst fluid CEA level >192 ng/mL was the most accurate marker for predicting the presence of a mucinous cyst. 7
The differential diagnosis of pancreatic cysts is becoming more common with the ever-increasing reliance upon radiologic imaging for the evaluation of patients. Numerous types of cysts can occur in the pancreas, including serous cystic neoplasms, mucinous cystic neoplasms (MCN), intraductal papillary mucinous tumors (IPMT), and pseudocysts. Prior studies have documented the considerable malignant potential of MCNs and IPMTs.6 Therefore, accurate diagnosis of pancreatic cysts is of paramount importance. Endoscopic ultrasound allows the operator to aspirate fluid from pancreatic cysts for cytology as well as amylase and CEA levels to help distinguish mucinous cysts from cysts with no malignant potential. The large Cooperative Pancreatic Cyst Study demonstrated that a cyst fluid CEA level >192 ng/mL was the most accurate marker for predicting the presence of a mucinous cyst. 7
Surgical resection of mucinous cystic neoplasms and intraductal papillary mucinous tumors can help prevent the onset of pancreatic cancer. Studies are ongoing to identify additional markers, such as specific DNA mutations, to help in the diagnosis of premalignant cysts. Furthermore, work is underway to assess the feasibility of treating cysts through ablation by EUS-guided injection of ethanol and other agents into the cyst cavity. Eventually it may be possible to eliminate the malignant potential of pancreatic cysts by therapeutic EUS, thus avoiding the need for major surgical procedures.
Multiple early studies clearly demonstrated that EUS provided superior imaging of the pancreas when compared to CT and MRI. 8 However, more recent studies have found little difference between EUS and CT or MRI for the local and regional staging of pancreatic cancer. This is most likely due to technologic advances in cross-sectional imaging. However, EUS does allow gastroenterologists to sample pancreatic masses by fine needle aspiration, thus providing a definitive diagnosis to help direct future therapy. Just as in other types of cancers, EUS and cross-sectional imaging should be thought of as complementary, rather than competing, modalities for staging (Figure 3). Non-invasive imaging such as CT and MRI can more accurately evaluate for metastatic disease, while EUS can help investigate local and regional staging with particular emphasis on the major vessels adjacent to the pancreas. A variety of private companies are studying various chemotherapeutic agents that may be injected into pancreatic tumors under EUS guidance to provide targeted therapy as well. Endoscopic ultrasound can be equally important in the evaluation of patients with pancreatitis. EUS is more sensitive than MRI and regular ultrasonography in the detection of common bile duct stones and gallstones, respectively.9 All patients with acute pancreatitis of unclear etiology should be considered for EUS in order to document the presence of microlithiasis in the gallbladder that may have been the precipitating factor, which would necessitate cholecystecomy. Endoscopic ultrasound can identify certain features within the pancreas, such as hyperechoic foci or ductal changes, that can be used to diagnose chronic pancreatitis. Finally, in patients with chronic pancreatitis or pancreatic cancer who have pain due to involvement of the adjacent celiac plexus by their disease, gastroenterologists can perform celiac plexus blockade via EUS to provide longer lasting pain control.
Figure 3: EUS image of an approximately 2cm mass in the head of the pancreas (thin circular outline). The mass compresses the CBD (common bile duct, bold white arrow) resulting in this patient’s presenting symptom of jaundice.
 Stomach and Rectum
Stomach and Rectum
EUS provides excellent evaluation of the layers of the gastrointestinal tract’s wall, thereby making EUS the first-line modality to investigate non-mucosal lesions of the GI tract. In the stomach, a variety of lesions can be diagnosed including gastrointestinal stromal tumors and carinoids (Figure 4). EUS also provides the most accurate modality for local staging of malignant lesions in the stomach, such as adenocarcinoma, lymphoma and MALToma (Mucosa Associated Lymphoid Tissue, a Non Hodgkins Lymphoma).10 Accurate staging of gastric cancer can identify which patients should undergo neoadjuvant therapy, thus improving long term survival. The greatest utility for EUS in gastric adenocarcinoma may be in patients with very early disease, in whom endoscopic therapy, without surgical intervention, may be curative.
Figure 4: EUS image of a nonmucosal mass seen on endoscopy. The mass was seen arising from the muscularis propria, consistent with a gastrointestinal stromal tumor. In this image, an FNA needle is seen penetrating the mass in order to obtain a sample for diagnosis.
 EUS plays a crucial role in the management of rectal cancer as well. Staging of rectal cancer by endoscopic ultrasound is straightforward and has been consistently shown to be more accurate than CT or MRI. 11 Neoadjuvant chemoradiation is the standard of care in patients with T3 disease as well as in those with lymph node involvement. 12 Preoperative treatment has been associated with improved local recurrence rates compared with postoperative therapy. CT understages as many as 39% of patients with rectal cancer who would have required neoadjuvant therapy for optimal management if properly staged.
EUS plays a crucial role in the management of rectal cancer as well. Staging of rectal cancer by endoscopic ultrasound is straightforward and has been consistently shown to be more accurate than CT or MRI. 11 Neoadjuvant chemoradiation is the standard of care in patients with T3 disease as well as in those with lymph node involvement. 12 Preoperative treatment has been associated with improved local recurrence rates compared with postoperative therapy. CT understages as many as 39% of patients with rectal cancer who would have required neoadjuvant therapy for optimal management if properly staged.
Experience at Lancaster General Hospital
From August 2009 through April 2010, 104 endoscopic ultrasound procedures were performed through Regional Gastroenterology Associates of Lancaster. The most common indications included staging or diagnosis for suspected pancreatico-biliary malignancy (n=22), staging of esophageal cancer (n=12), investigating pancreatic cysts (n=12) and evaluating patients with a dilated common bile duct (n=10). No complications occurred in any patients that underwent EUS. When performed for a solid mass or suspicious lymph node, fine needle aspiration had a diagnostic yield of 77% (n=35). Furthermore, EUS altered the clinical management of patients in 73 out of 104 cases. Most commonly, EUS directed the administration of appropriate neoadjuvant therapy prior to possible surgical resection of a malignancy.
The power of endoscopic ultrasound can be illustrated by the recent case of a patient who had been in remission for three years after receiving chemotherapy for non small cell lung cancer. He now presented with new lesions in his liver as well as in the uncinate process of the pancreas. The differential included either recurrent lung cancer or newly metastatic pancreatic cancer. Neither the liver or pancreatic lesion was amenable to percutaneous biopsy. However, through EUS we were able to sample the pancreatic lesion and confirm recurrent lung cancer, thus providing a tissue diagnosis and helping to direct the appropriate chemotherapeutic regimen.
Conclusion
Endoscopic ultrasound has an important role in the staging of a variety of cancers and in the evaluation of a myriad of benign gastrointestinal diseases. Currently, endoscopic ultrasound has its greatest impact on the management of esophageal, non-small cell lung, pancreatic, gastric, and rectal cancers. The identification of patients appropriate for neoadjuvant chemotherapy and radiation therapy has been shown to have a positive impact on recurrence-free survival after surgical resection of a number of cancers. It is important to realize that EUS has many more indications beyond staging of malignancy, from evaluating the etiology of pancreatitis to investigating pancreatic cysts. Furthermore, the role of EUS will continue to expand as the technology advances. The ability to introduce FNA needles via endoscopic ultrasound allows for not only the ability to sample suspicious lesions, but also provides the opportunity for therapeutic interventions as well. In the future, EUS may be used to ablate premalignant pancreatic cysts or to locally treat pancreatic cancer. Greater insight into endoscopic ultrasonography will allow physicians to utilize EUS in the appropriate settings, thus having the greatest clinical impact on their patients.
REFERENCES
1Rosch T. Endosonographic staging of esophageal cancer: A review of literature results. Gastrointest Endosc Clin North Am 1995; 5:537-547
2Harewood GC, Kumar KS: Assessment of clinical impact of endoscopic ultrasound on esophageal cancer. J Gastroenterol Hepatol 19:433-439, 2004.
3Hawes RH and Fockens P. Endosonography. Philadelphia: Elsevier Inc., 2006.
4Fritscher-Ravens A, Davidson BL, Hauber HP et al. Endoscopic ultrasound, positron emission tomography, and computerized tomography for lung cancer. Am J Respir Crit Care Med 2003; 168:1293-1297.
5Wallace MB, Pascual J, Raimondo M et al. Minimally invasive endoscopic staging of lung cancer. JAMA 2008; 299 (5): 540-546
6Zamboni G, Scarpa A, Bogina G, et al. Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am J Surg Pathol 1999; 23 (4): 410-422
7Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology 2004; 126: 1330-1336
8Muller MF, Meyenberger C, Bertschinger P et al. Pancreatic tumors: evaluation with endoscopic US, CT and MR imaging. Radiology 1994; 190:745-751
9Lambert R. Clinical outcome of EUS in biliary diseases. Endoscopy 2000; 32:558-561
10Kelly S, Harris KM, Berry E et al. A systematic review of the staging performance of endoscopic ultrasound in gastro-oesophageal carcinoma. Gut 2001; 49:534-539
11Kwok H, Bisset IP, Hill GL. Preoperative staging of rectal cancer. Int J Colorectal Dis 2000; 15:9-20.
12National Institutes of Health consensus conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA 1990; 264:1444-1450