 Click to Print Adobe PDF
Click to Print Adobe PDF
Winter 2010 - Vol.5, No.4
  Bradburn Frankel
Bradburn Frankel
Diagnosis and Management of Splenic Trauma
Eric H. Bradburn, D.O
Acute Care Surgeon, Lancaster General Hospital
Heidi L. Frankel, M.D., FACS, FCCM
Chief of the Department of Trauma, Critical Care, and Acute Care Surgery, Penn State Milton S. Hershey Medical Center
|
|
|
ABSTRACT
The diagnosis and management of splenic trauma has evolved over the past several decades. The spleen, once thought expendable, is now viewed as a vital component of the immune system. Adult trauma surgeons have learned from their pediatric counterparts that non- operative management is possible even with higher grade injuries; interventional radiology has increased successes with non-operative approaches. Improvements in assessment of injuries with adjuncts such as the FAST exam and higher resolution CT scanners have allowed reliable identification of variables that can guide the surgeon either to immediate laparotomy, angiography, or a non-operative course. We outline in this review the evaluation, assessment, and management of splenic trauma. The current standard of non-operative management is discussed and demonstrated by the protocol used at Lancaster General Hospital. We also describe the technique of splenectomy and its complications.
HISTORICAL CONTEXT
The great ancient Roman physician Galen described the spleen as “Plenum mysterii organum” or “the organ full of mystery” as he struggled to elucidate its function. The mystery continued for over a millennium, as no one challenged his theory that the spleen functioned to remove the evil humor “black bile” produced by the liver.1 As understanding of physiology and anatomy improved, we learned that the spleen stored and removed aging red cells and platelets and produced opsonins, properdin, and tuftsin. East Indian criminals dubbed “Thugees” certainly appreciated the spleen as a storage site for cells, as they aimed to permanently incapacitate their victims with a blow to the left upper quadrant. Nonetheless, the presumed expendability of the spleen guided therapy during much of the last century, when marginally positive diagnostic peritoneal lavages frequently resulted in mandatory laparotomy and splenectomy.
It appears that we have come full circle over the last several decades. Splenic salvage is once again considered appropriate, reinforced by the pediatric experience with successful non-operative management (NOM), an understanding of the intact spleen’s vital role in healthy immune function, and the use of more detailed diagnostic modalities to further delineate the extent of injury. 2
PATHOPHYSIOLOGY
Indeed, the intact spleen deserves respect, but the damaged spleen warrants even greater reverence. The spleen is top of the list of organs most injured in blunt trauma and, if the injury is unrecognized or not skillfully managed, the patient with splenic trauma can rapidly exsanguinate. In the past, 40-50% of injured spleens required operative intervention, most likely due to confusion regarding splenic function, as well as about diagnosis in the pre-CT era. We certainly recognize the importance of the spleen in the pediatric population where NOM has been a longtime norm and overwhelming post-splenectomy sepsis (OPSI) is more problematic. However, in the adult trauma population, the development of NOM was a slow evolutionary process and it is one that continues to advance.
Trauma surgeons have learned much about the spleen from their pediatric colleagues. At the turn of the century, the non-operative approach to all splenic injuries carried a 90-100% mortality. Today, 95% of splenic injuries in the pediatric population are successfully managed non-operatively. In the adult population, the numbers are substantially lower, with 60% of all splenic injuries managed non-operatively, with a failure rate of about 10%.3 The Memphis group reports an observation rate of 77% with a failure rate of 8%.4
What explains the divergent experience of NOM in pediatric and adult populations? Foremost, the pediatric patient is physiologically different from the injured adult. The physiologic reserve of a child is far greater than that of a 65 year old with multiple medical co-morbidities that may render a surgeon reluctant to pursue NOM. Additionally, the splenic capsule and parenchyma in children is much firmer and tolerant of greater injury. Finally, children are simply injured in a different fashion than are adults. Powell and colleagues demonstrated that adults had higher Injury Severity Scores (ISS) and lower Glascow Coma Scores (GCS) than children, and were more likely to sustain multiple injuries. In addition, transfer of the kinetic energy of injury is different between adults and children, as a result of different mechanisms of injury and the lower mass of a child.5
EVALUATION
The initial evaluation and management begins with a high index of suspicion based on mechanisms of injury. The spleen is nestled in the left posterior upper quadrant in intimate contact with the diaphragm, stomach, pancreas, and colon. It is particularly vulnerable to trauma of the left lower thorax. One must consider the presence of associated injuries to the diaphragm, pancreas, and bowel. Associated injuries can be an undue source of excessive morbidity and mortality and can readily be ruled out by immediate laparotomy. Failure to recognize an associated injury can prove catastrophic to the patient. Much consideration is needed in cases of trauma in elderly patients as they may initially present with few findings due to altered physiology as a result of medications such as beta blockers. Elderly patients are also more commonly taking anticoagulants such as aspirin, clopidogrel, and warfarin, which may result in delayed hemorrhage and failure of attempts at splenic preservation.
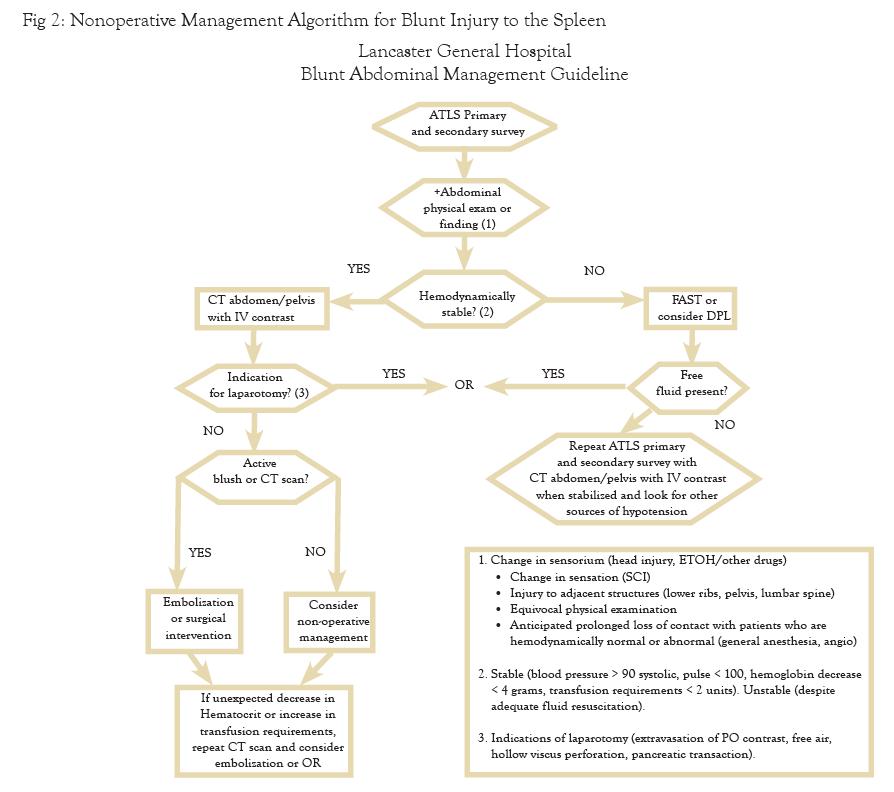
The most important consideration guiding evaluation for splenic injury is the patient’s hemodynamics. If the patient has a normal pulse rate and blood pressure, then workup can proceed to CT scanning with IV contrast to determine whether a “blush” of “extravasated contrast” is present. Active extravasation correlates with ongoing arterial bleeding, and it is unwise to ignore that finding, as up to 2/3 of adults and 1/3 of children will fail such a management strategy. Active contrast extravasation should prompt either coil embolization by interventional radiology, or operative intervention, depending on institutional resources and continued patient stability. The CT scan also establishes the grade of splenic injury, the amount of hemoperitoneum (HP), and associated injuries, all of which play an important role in determining whether the patient might require operative intervention. In the unstable patient, the algorithm utilizes Focused Assessment with Sonography for Trauma (FAST) and Diagnostic Peritoneal Aspiration (DPA) to determine the presence of intra-abdominal blood and, ultimately, the need for operative intervention.
INJURY ASSESSMENT
The value of injury grade in stratifying patients between operative and NOM is not entirely reliable. The most accepted grading scale for splenic injury was established by the American Association for the Surgery of Trauma in 1987 and revised in 1997 (FIGURE 1). In general, the lower the injury grade the more likely the patient can be managed non-operatively. However, the CT scan is notorious for underestimating injury grade,6 so injury grade alone should not guide the surgeon. In addition, even higher grade injuries may be amenable to NOM, particularly with liberal use of angioembolization (AE).3 In patients who are hemodynamically stable and have a “blush,” AE should be considered if institutionally available.
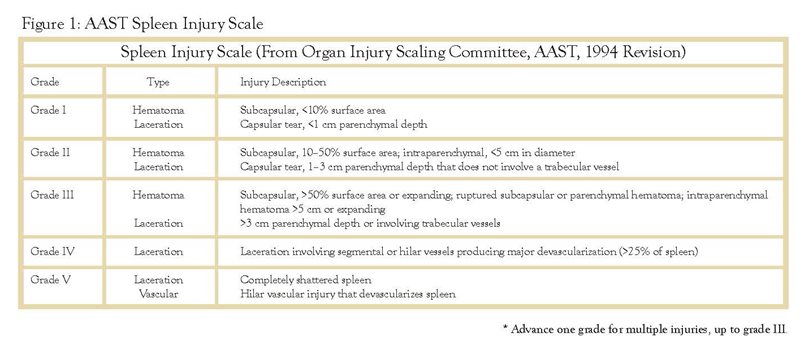
The amount of hemoperitoneum (HP) can also be useful to determine the approach to the patient with an injured spleen. Multiple authors have suggested that the presence and quantity of free fluid in the post traumatic abdomen can predict the need for operative intervention. In the large multi institutional spleen study conducted by the Eastern Association of the Surgery of Trauma published in 2000, The size of HP made a significant difference in the proportion of patients successfully managed non-operatively. NOM was ultimately successful for 80.1% of patients with a small amount of blood versus only 27.4% with a large HP.3 Gonzales and associates reported the failure of NOM based on the quantity of HP to be 10%, 22%, and 48% for small, moderate, and large amounts of fluid, respectively.7 Similar conclusions were drawn by Sharma and colleagues in their patient populations with large HP. On the contrary, the Memphis group in their study of 558 patients with blunt splenic injury demonstrated that HP alone was not independently predictive of the need for operative intervention and should not be a contraindication for NOM.4
Advanced patient age may as also be a possible contraindication to NOM. The initial basis for this rationale was postulated from data that suggested higher failure rates and increased mortality for NOM in trauma patient groups over the age of 55. However, upon close examination of these data, the higher mortality rates were likely secondary to associated injuries and related post trauma complications. Bee and Cocanour both described high rates of success when NOM was employed in patients >55 years old. Cocanour compared 375 patients stratified into cohorts with 55 years of age as a cutoff (n=346 <55 and n=29 >55), and the failure of NOM did not significantly differ between the two groups (17% and 14% respectively). On the other hand, Godley and co-authors reported a 91% failure for patients >55 with splenic trauma.
Moreover, one must consider the increases in hospital and ICU length of stay (LOS), and potential complications in the elderly who fail NOM. Siriratsivawong and colleagues concluded that mortality was high regardless of failure of NOM, but significant increases in hospital stay and ICU LOS were found in those patients over 55 years of age. Fabian’s group states this point eloquently: “Consideration must be given to all aspects of the patient’s condition rather than dictating an axiom requiring all patients over age 55 to undergo operative management.”
NON OPERATIVE MANAGEMENT (NOM)
The decision to employ the NOM pathway for blunt splenic injury requires the patient to meet several criteria. The first and foremost is hemodynamic stability with the absence of any suspected associated intra-abdominal injury. Although many of the developed pathways and institutional protocols that address NOM of splenic injury include contraindications such as age ranges, splenic grades, transfusion triggers and quantities, the remainder of exclusionary criteria are more individualized than strict convention. Certainly there are several clear absolute contraindications which include the patient who is receiving or will receive systemic anticoagulation. Special consideration is in order for injured pregnant women with viable preterm fetuses who would not tolerate the stress of NOM failure. Also the patient with multiple injuries or traumatic brain injury with a mid to high grade splenic injury poses a particular challenge to NOM.
The remaining factors in the decision rely on sound clinical judgment and good surgical common sense. Most clinicians are now aware of the immunosuppressive effects of blood transfusion, acknowledge the harmful effects of stored blood, and recognize that transfusion practices should be based on physiology rather than a number. In addition, we have learned much concerning the inflammatory cascade, which - if unchecked - can cause undue morbidity and mortality. Indeed, the trauma surgeon must learn to balance the risks of bleeding and transfusion with the morbidity of an increased splenectomy rate.
Successful angioembolization can eliminate the need for operative intervention even for many high grade splenic injuries. Scalfani and associates reported an 84% salvage rate with the use of coil embolization for Grade IV splenic injury, and in other series up to 2/3 of Grade IV splenic injuries could be managed in this fashion. There are probably insufficient data about the success of this strategy for Grade V injuries.
The choice of NOM mandates the use of a management pathway based on a protocol. Though there is little evidence yet in the literature to substantiate many of the recommendations in institutional protocols, success with NOM requires firm clinical guidelines. Protocols should address several key questions:
a) Where should the patient be admitted (based on grade level)
b) How long should the observation period last?
c) How often should the hematocrit be assessed?
d) How long should anticoagulation be interrupted?
e) When/if to follow up with imaging?
f) When should vaccination be given?
The question of where to admit should be based on injury grade. It is our institutional practice to admit all injuries grade III or above to the intensive care unit. Grade I and II injuries can be admitted to a less intensive monitored setting. Certain grade I injuries may not require admission and observation, but always while taking into account that CT is notorious for underestimating injury. The period of observation is debatable and the clinical condition and progress of the patient should play a role in deciding duration. Multiple studies have concluded that most failures of NOM occur in the first 72 hours of admission. Smith and colleagues suggest that if hematocrit and pulse are stable after 48 hours, then patients can be ambulated and fed.8 The hematocrit should be checked frequently for high grade injuries and less frequently for grade I and II injuries. There is no clear consensus in the literature about a specific number of transfusions as a requirement for determining need for an operation. Given the infectious risks associated with stored blood products, our experience recommends that the transfusion requirement should not exceed two units of packed red blood cells. Our institutional pathway is found in FIGURE 2.
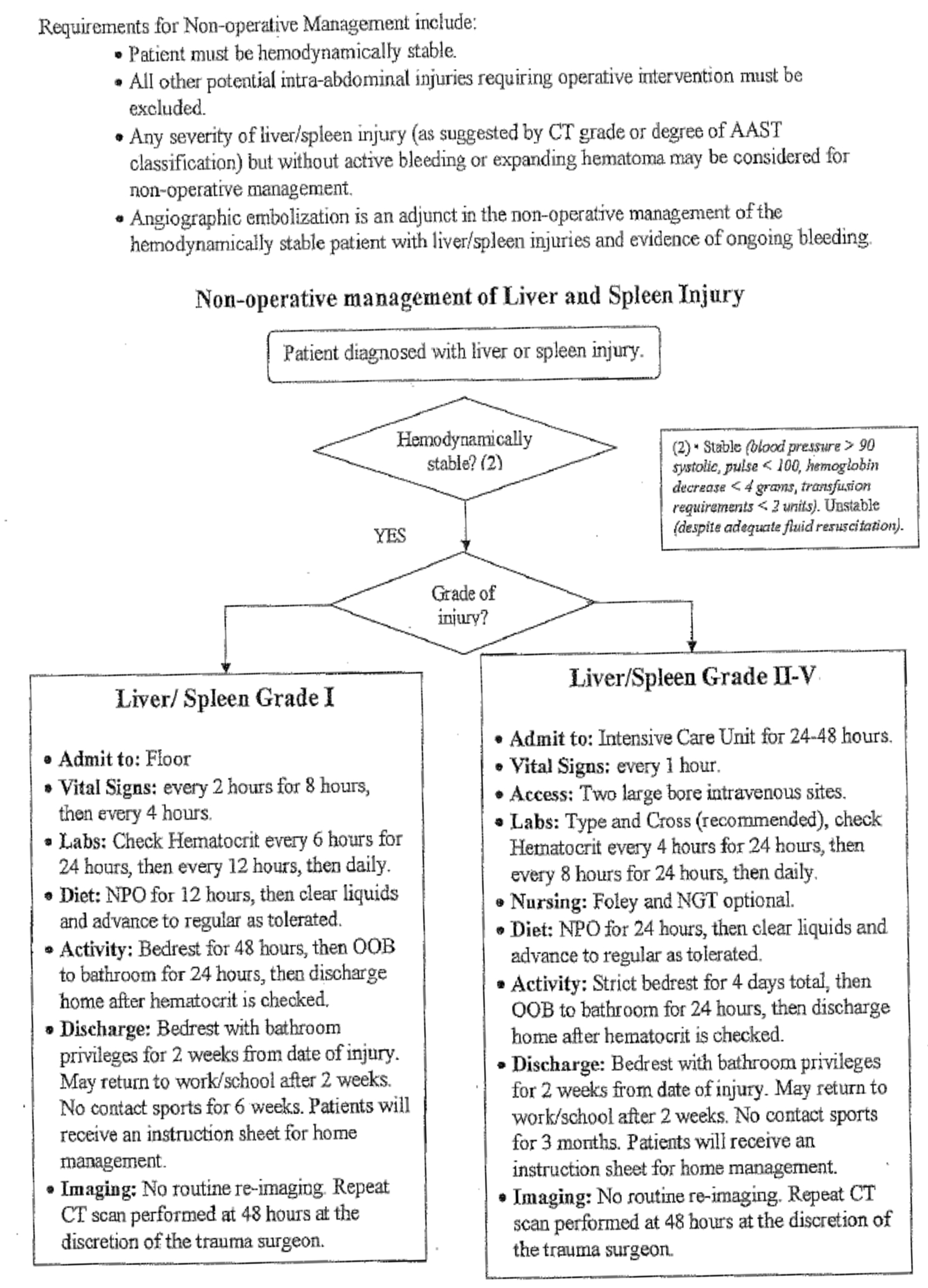
The issue of limitation of activities is a reasonable consideration, but it must be balanced against the risk of potential complications caused by inactivity. The use of follow-up imaging is controversial, and there is no general consensus on the utility of repeat CT scans. Shurr and co-authors reported that splenic artery pseudo-aneurysms increase the failure rate of NOM, so it may make sense to rescan individuals with higher grade splenic injuries. In fact, Weinberg confirms this practice by utilizing serial CT to identify pseudo-aneurysms and prompt subsequent embolization. This resulted in a 97% splenic salvage rate in 341 patients over a 2 year study period.9 Vaccination is essential after splenectomy, but it is unclear whether the patient with a high grade splenic injury managed non-operatively requires prophylactic vaccination.
OPERATIVE MANAGEMENT
The evaluation of the hemodynamically unstable patient proceeds down a simpler pathway with the goal of ruling in or out a trip to the O.R. or angiography suite. Rapid resuscitation techniques are applied to the unstable patient while simultaneously attempting to rapidly identify the source of hemorrhage.
The credo that states “all unstable trauma patients are in hemorrhagic shock until proven otherwise” serves the trauma surgeon well. In this arena of shock, the blood is either in the chest, abdomen (pelvis and retroperitoneum), thighs, or on the floor. In the unstable patient, the sonographic exam (FAST) has proven an excellent tool to evaluate the presence or absence of intra-abdominal fluid and has largely replaced diagnostic peritoneal aspiration (DPA). An unstable patient with a positive FAST warrants urgent laparotomy. If the FAST is negative and the patient remains unstable, one must still suspect occult bleeding within the abdomen. In these cases, DPA can be utilized to further guide the clinician to the potential source of shock. The DPA is considered positive with 10ml of frank blood, which mandates laparotomy. Approximately 40% of patients with splenic injuries will present with hemodynamic instability and require immediate laparotomy. A subset of patients will arrive hemodynamically stable with reported transient hypotension in the field or during transport, a finding that should be respected and should heighten suspicion of ongoing hemorrhage.
Splenectomy should be considered for the patient with an injured spleen who is clinically unstable (FIGURE 3). The injured spleen should also be removed in the case of devascularization, coagulopathy, traumatic brain injury (TBI), and in patients with multiple other sources of bleeding. Exposure is the key to success in surgery and the spleen is no exception. The best incision is the midline approach because it is not only the most versatile, but is clearly the best to rule out injuries elsewhere in the abdomen. After thorough exploration of the entire abdomen, the spleen is mobilized and elevated into the operative field. If severely damaged, it is removed after securing its blood supply with careful attention to avoiding injury to the tail of the pancreas.
If the spleen has sustained only minimal damage there are several options for splenic salvage. These include partial splenectomy or a mesh wrapped repair. These techniques are now sparsely applied, especially in the case of failed NOM, as the patient will be at too great a risk if failure of NOM is followed by failure of surgical salvage of the spleen; splenectomy avoids these risks. Coincidentally, the spleen seems especially friable in these cases and the usual techniques to accomplish salvage, such as omental patches, pledgeted repairs, or stapled resections, are often unwise. Additionally, salvage may not be necessary as many have theorized that patients who sustain splenic trauma have some splenic function sustained by splenosis - ectopic splenic tissue that is acquired as a result of seeding the peritoneum with fragments of viable splenic tissue from either traumatic or iatrogenic causes.
POST SPLENECTOMY COMPLICATIONS
Many studies have established the increased risk of infection in splenectomized patients, with particular susceptibility to pneumococcal, meningococcal, and Haemophilus infections. Shatz reported that 99.2% US and Canadian trauma surgeons surveyed vaccinate their splenectomized patients,10 though only 56% routinely gave all three vaccinations. Although the incidence of Overwhelming Post Splenectomy Infection (OPSI) is low in the adult population, its mortality exceeds 50%. In addition, there is a considerable literature on the increased risk of general infections in this cohort. It therefore makes sense to vaccinate this population with all three vaccines in a standard of care fashion. The timing of vaccines has been debated, but Schreiber established in an animal model that pneumococcal vaccination within 24 hours post splenectomy reduces mortality.11 In our practice we administer all vaccines within one week post operatively to balance the need for immune competency with that of ensuring administration. Since OPSI is extremely rare in the adult population, prophylactic antibiotics are only recommended in patients less than 4 years of age.
The other complications of splenectomy include bleeding, infection, pancreatic fistula, thrombosis, thrombocytosis, and death. Overall morbidity is around 4-10%. Pancreatic fistula (PF) can be an early or late complication of splenectomy and lead to local infection, pseudocyst formation, and sepsis. The true incidence of PF post splenectomy is not defined. The essence of treatment involves externalizing the fistula via a drain.
CONCLUSIONS
In managing splenic trauma, an assessment of the overall clinical picture is clearly more important than any specific aspect of evaluation. The era of pan CT scanning has afforded the trauma surgeon the luxury of carefully selecting patients for NOM. This development is clinically important as the literature is filled with reports about the morbidity of the non-therapeutic laparotomy. We also have a greater understand of transfusion,the adverse consequences of transfusion, and the morbidity associated with stored blood products. Though this understanding might lead some surgeons to early laparotomy, in the end the surgeon must be guided either toward the O.R. or toward NOM by evidence-based practice combined with common sense.
The mysteries of the “organ of black bile” still challenge us in this modern age. However, as opposed to Galen’s time when no one dared challenge his great mind, we have learned to challenge each other to elucidate the mysteries that lie within our practices of surgery.
REFERENCES
1. Nuland S. The Mysteries Within: A Surgeon Explores Myth, Medicine, and the Human Body. New York, NY: Touchstone, 2000.
2. Swirski FK, Nahrendorf M, Etzrodt M, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009; 325:612-6.
3. Peitzman AB, Heil B, Rivera L, et al. Blunt splenic injury in adults: Multi-institutional Study of the Eastern Association for the Surgery of Trauma. J Trauma 2000; 49:177-87; discussion 187-9.
4. Bee TK, Croce MA, Miller PR, Pritchard FE, Fabian TC. Failures of splenic nonoperative management: is the glass half empty or half full? J Trauma 2001; 50:230-6.
5. Powell M, Courcoulas A, Gardner M, et al. Management of blunt splenic trauma: significant differences between adults and children. Surgery 1997; 122:654-60.
6. Shapiro MJ, Krausz C, Durham RM, Mazuski JE. Overuse of splenic scoring and computed tomographic scans. J Trauma 1999; 47:651-8.
7. Gonzalez M, Bucher P, Ris F, Andereggen E, Morel P. [Splenic trauma: predictive factors for failure of non-operative management]. J Chir (Paris) 2008; 145:561-7.
8. Smith JS, Jr., Cooney RN, Mucha P, Jr. Nonoperative management of the ruptured spleen: a revalidation of criteria. Surgery 1996; 120:745-50; discussion 750-1.
9. Weinberg JA, Magnotti LJ, Croce MA, Edwards NM, Fabian TC. The utility of serial computed tomography imaging of blunt splenic injury: still worth a second look? J Trauma 2007; 62:1143-7; discussion 1147-8.
10. Shatz DV. Vaccination practices among North American trauma surgeons in splenectomy for trauma. J Trauma 2002; 53:950-6.
11. Schreiber MA, Pusateri AE, Veit BC, Smiley RA, Morrison CA, Harris RA. Timing of vaccination does not affect antibody response or survival after pneumococcal challenge in splenectomized rats. J Trauma 1998; 45:692-7; discussion 697-9.