Click to Print Adobe PDF
Fall 2009 - Vol.4, No.3
A New Tool to Help Fight Cancer—Tomotherapy
Jeffrey S. Eshleman, M.D.
Lancaster Radiology Associates, Ltd.
|
 |
Abstract
Advances in radiation therapy for cancer are reviewed, with emphasis on TomoTherapy® Hi•Art,® a new system for delivering therapy with a helical delivery system in which the patient and the radiation source move in a manner similar to that used for diagnostic CT scans. Simultaneously, the intensity of the radiation beam is modulated by a computer controlled mechanism. The Lancaster General Cancer Center now offers this technology in a new state-of-the-art center. (Fig. 1) The first patient was treated on June 2, 2009. This report summarizes the advantages, disadvantages, and applicability of this device.
Figure 1. The new TomoTherapy® Hi•Art® radiation therapy delivery system at the Lancaster General Cancer Center.
INTRODUCTION
Radiation therapy has been used as a cancer treatment for more than 100 years, beginning shortly after the discovery of x-rays in 1895 by Wilhelm Roentgen (Röntgen in the original German).[1]
Radiation is still one of the most effective cancer treatments available today. Radiation oncologists make use of highly sophisticated, precise, and intricate instruments, such as stereotactic devices, linear accelerators, brachytherapy, and radiopharmaceuticals, to precisely target tumors with ionizing radiation.
During the past 50 years, the most common form of radiotherapy has been external beam radiation therapy (EBRT). Conventional EBRT uses a beam of high energy x-rays or photons directed at the tumor from one to several different directions, so that radiation is focused on a target and surrounding healthy tissue is shielded. EBRT is typically delivered via a linear accelerator, a machine which produces and focuses photons towards the target, with photons exerting their effects by ionization of molecules in tissues directly within their path (Figure 2).

| Figure 2: A 6-18MV conventional linear accelerator at Lancaster General Cancer Center. The linear accelerator directs high energy photons in a step and shoot fashion towards a target as demonstrated by the blue beam. At bottom left is a close up view of the multi-leaf collimator (MLC, blue arrow) which resides in the head of the machine. The leaves of an MLC shape the beam. In IMRT, the leaves continuously move in complex geometric patterns to modulate the intensity of the beam. This results in highly conformal distributions of radiation dose. |
The formation of thousands of intracellular free radicals by ionization, notably hydroxyl radicals, damages the DNA by binding to the DNA bases and causing single and double stranded DNA breaks.[2] DNA damage is then inherited through cell division. Normal cells have intact mechanisms for repairing DNA damage, while cancer cells generally have a diminished ability to repair such sub-lethal damage, so a therapeutic window is generated that is enlarged by fractionating radiotherapy treatments.
At first, it was very difficult for therapeutic radiologists to deliver enough radiation to kill a tumor without causing painful or debilitating side effects. Even though normal tissue can recover from exposure to radiation, there are often side effects, and too much radiation can damage normal tissue beyond repair.
Significant improvements have been made with conventional EBRT during the past 50 years. As technology has progressed, the ability of radiation oncologists to conform radiation to their targets while minimizing dose to normal tissues has greatly increased, and as a result, treatment efficacy and tolerability have greatly improved. By increasing the number of beams focused on the target and the eventual ability to shape these beams with simple and later complex blocking devices, radiation plans became more conformal.
Despite these improvements, however, treatment plans continued to have unwanted high doses of radiation well outside the intended target. Over the past two decades a novel, highly sophisticated technology, called intensity-modulated radiation therapy (IMRT), has been developed which allows precise coverage of targets regardless of size or shape.
INTENSITY-MODULATED RADIATION THERAPY (IMRT)
In IMRT, very small beams, or beamlets, are aimed at a tumor from many angles. During treatment, the radiation intensity of each beamlet is controlled by a device called a multileaf collimator (MLC) (Figure 2, left lower corner). The collimator is a computer-controlled mechanical device that modulates the shape and amount of radiation delivered by blocking out some areas and filtering others through movement of the leaves. The beam's intensity is thus modulated to distribute the intended radiation dosage precisely in complex shapes and patterns. As a result, the radiation dose is shaped to the tumor geometry and bends around important healthy tissues in a manner that would be impossible with conventional radiation techniques.[3]
Conventional linear accelerators were adapted to perform IMRT utilizing a stop, point and shoot approach to delivery. Each beam is focused from a specific direction, with each beam highly modulated by the computer controlled MLC as discussed above.
TOMOTHERAPY
Recently, TomoTherapy Inc. developed a novel approach to IMRT. While conventional radiation therapy machines have been adapted and re-adapted to keep up with advances in the field, the new TomoTherapy unit was designed without legacy constraints.
TomoTherapy® Hi·Art® system is a new, advanced, state of the art, helical IMRT delivery system with CT image guidance. (Figure 2) The system was developed at the University of Wisconsin-Madison and the first patient was treated in 2003.[4]
TomoTherapy® Hi•Art® shares similar technology with CT scanners, otherwise known as computerized tomography. A small megavoltage x-ray source was mounted in a fashion similar to that of a CT x-ray source, and the geometry provided the opportunity to provide treatment utilizing the continuous spiral 360 degree rotation of the CT gantry (Figure 3). High energy photon radiation is produced by the mounted megavoltage linear accelerator, which travels around the gantry ring in a spiral fashion.
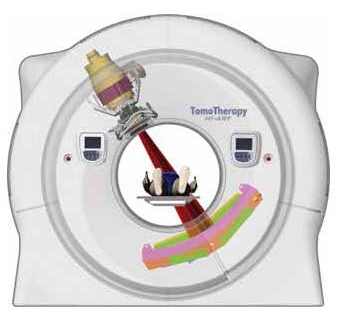
Figure 3: The TomoTherapy® Hi•Art® ™ system delivers radiation through a small megavoltage x-ray source mounted in a similar fashion as a CT x-ray source. Radiation is delivered in a continuous spiral 360 degrees rotation. (Image reproduced with permission from TomoTherapy® Inc.) |
The linear accelerator moves in unison with a MLC. The MLC has two sets of interlaced leaves that move in and out very quickly to constantly modulate the radiation beam as it leaves the accelerator. Meanwhile, the couch is also moving, guiding the patient slowly through the center of the ring, so each time the linear accelerator comes around, the beam is directed at a slightly different plane (Figure 4).
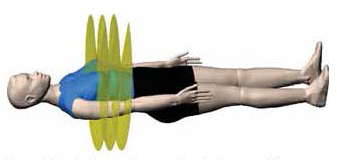
Figure 4: The patient slowly moves through the center of the gantry, so each time the linear accelerator comes around, the beam is directed at a slightly different plane. Radiation dose is modulated at every angle to conform to the target. (Image reproduced with permission from TomoTherapy® Inc.) |
Typically, tens of thousands of beamlets are included in one treatment session. A single beamlet corresponds to the radiation emitted through an open leaf of the MLC with the gantry at any given angle, during any given rotation. TomoTherapy® Hi•Art® offers by far the most beamlets of any form of IMRT, which translates to one of the most precise conformal radiotherapy available.
IMAGE GUIDANCE RADIATION THERAPY (IGRT)
For any conformal radiotherapy system to be effective, the anatomical position of the tumor and surrounding healthy tissues must be accurately defined and localized. TomoTherapy® Hi•Art® has the ability to perform a quick CT scan before each treatment starts, to ensure the patient is aligned accurately with the treatment plan (Figure 5).
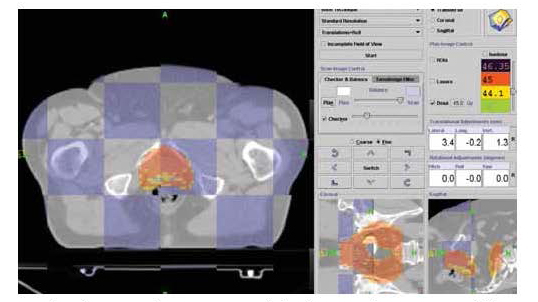
| Figure 5: Daily CT image of prostate cancer patient fused with treatment planning CT. Corrected shifts are made to ensure the patient is accurately aligned prior to treatment. |
In addition to localizing the target, physicians can also monitor changes in tumor volume and anatomy during the treatment course. For example, when a tumor responds to treatment over a several week treatment course, changes in tumor anatomy or in surrounding tissues can occur. If not recognized, the target volume may shift outside of the originally planned target, resulting in a geographic miss, potentially compromising local control or cure. With TomoTherapy® Hi•Art®, review of CT imaging ensures that geographic misses do not occur.
CLINICAL USES OF TOMOTHERAPY® HI•ART® RADIATION THERAPY SYSTEM
TomoTherapy® Hi•Art® can be used to treat tumors of various sizes, single or multiple tumors, one region of the body or several regions, to the same dosage in every area or to multiple different radiation dosages.
TomoTherapy® Hi•Art® is currently being used to treat select tumors in the prostate, brain, head and neck, lung, liver, pancreas, prostate, uterus as well as other sites. One of the most exciting aspects of the TomoTherapy® Hi•Art® system is that tumors can be treated radiosurgically.
RADIOSURGERY
Radiosurgery, also known as stereotactic body radiotherapy (SBRT) when used to target lesions in the body, is radiation treatment that delivers a very high dose of radiation in one to five fractions with great precision and localization. Unlike conventional fractionated radiotherapy, which may require as many as 25-35 daily fractions, with SBRT patients receive only one to five fractions. SBRT has been used to treat tumors of the lung, pancreas, liver, prostate, spine, head and neck, as well as benign tumors. For centers without an intracranial stereotactic radiosurgery technology, TomoTherapy® Hi•Art® may be used to deliver single or multiple fraction stereotactic radiosurgery (SRS) of intracranial lesions as well.
Recently, several studies of SBRT for medically inoperable Stage I non-small cell lung cancer have been reported.[5][6][7][8] Patients received 4-6 fractions of SBRT to a total dose of 40-60 Gy. The local control rate ranged from 80-95% at 3 years with very little treatment related morbidity. (About 10% of patients with intrathoracic lesions 10% have some signs of radiographic pneumonitis.)
An example of radiosurgery for a Stage I non-small cell lung cancer is seen in figure 6. At this writing (July, 2009) only short-term follow-up is available. The patient tolerated treatment well, suffered no acute toxicities from treatment, and is doing well thus far. It can take from 1 - 6 months to demonstrate a complete radiographic response.
Typically, a re-staging CT chest study would be performed 2-3 months post-treatment.
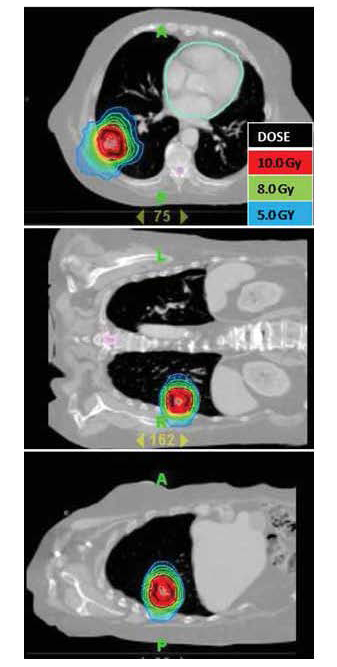
| Figure 6: Medically inoperable patient with Stage I non-small cell lung cancer. The lesion is seen in three views. The patient received SBRT to a total dose of 10.0 Gy times 5 fractions. |
TOMOTHERAPY AND PROSTATE CANCER
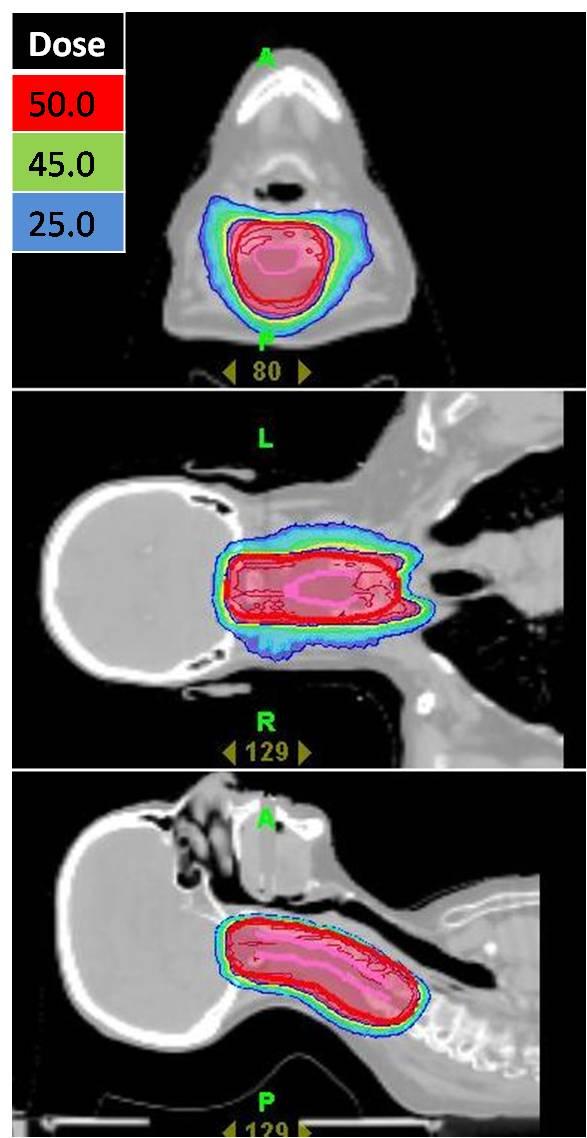
Radiotherapy (RT) is one of the established primary modalities for treating prostate cancer. Approximately 30% of all prostate cancer patients who are treated with curative intent receive RT, most with external beam radiotherapy, and a substantial proportion will be cured.[9][10] Studies have shown that IMRT/IGRT allows significant escalation of radiation dose with excellent biochemical control of disease with the same or less treatment-related morbidity.[11][12] With theTomoTherapy® Hi•Art® system, radiation is highly conformed to the prostate, minimizing dose to surrounding bowel, bladder and hips (Figure 7). Daily CT Image guidance ensures the prostate is exactly localized prior to treatment.
An exciting new development in the treatment of prostate cancer is the potential for hypofractionated radiotherapy.[13][14][15][16][17][18] Currently prostate patients are required to attend daily treatments, 5 days a week, over a treatment course of 7-8 weeks. Treatment can be costly and disruptive for patients, especially those not living close to a cancer center. Recent improvements in technology, such as TomoTherapy® Hi•Art,® allow high doses of radiation to be delivered to the prostate in fewer fractions while maintaining lower doses to adjacent bladder and rectum. This process is called hypofractionated stereotactic radiotherapy. Clinical trials are ongoing and further research is necessary before hypofractionated radiotherapy is offered outside of a clinical trial.
| Figure 7: A highly conformal radiation plan utilizing the TomoTherapy® Hi•Art® treatment planning software for a patient with early stage prostate cancer. In red, the high dose radiation precisely encompasses the prostate. There is a steep dose gradient (blue) surrounding the prostate. The dose of radiation delivered to the adjacent bladder and rectum is small, significantly reducing the potential for acute and/or chronic bowel or bladder complications. |
OTHER TUMOR SITES
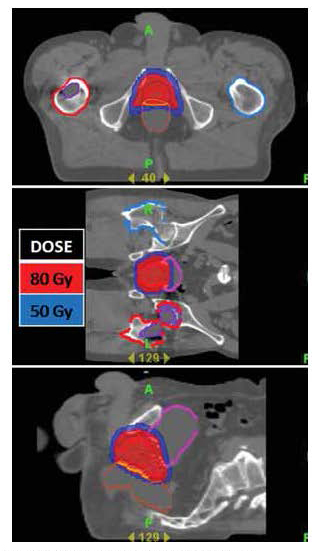
Many other patients may benefit from the Tomotherapy® Hi•Art® technology. Figure 8 shows a treatment plan for a patient with tumor involving the cervical spine. With TomoTherapy® Hi•Art® treatment, the full dose of radiation was delivered while sparing the esophagus, significantly reducing treatment morbidity. The patient did not develop an acute radiation mucositis/esophagitis. If conventional radiation techniques had been used, the likelihood of a grade 2 or 3 phayrngitis/esophagitis with the dose this patient received would have been >50%.
| Figure 8: A highly conformal radiation plan utilizing the TomoTherapy® Hi•Art® treatment planning software for a patient with a cervical spine tumor. With sparing the posterior pharynx and esophagus, the patient did not develop an acute radiation mucositis/esophagitis. |
POTENTIAL DISADVANTAGES OF IMRT/TOMOTHERAPY
IMRT and thus TomoTherapy® Hi•Art® may not be appropriate for all patients requiring radiotherapy. In IMRT, though the region that is exposed to high dose radiation is highly conformal, low doses of radiation are typically spread over a larger volume of normal tissue compared with conventional radiation treatments. Therefore, the total absorbed dose to the individual, called the integral dose, is much higher in patients receiving IMRT. As IMRT is a new technology, the absolute risks of a higher integral dose are not completely known at this time.
In general, most tissues tolerate low doses of radiation well and the risk of acute or chronic damage to exposed normal tissues is very small. However, following exposure to low dose radiation there is a slightly increased risk of radiation-induced second malignancies years or even decades following treatment.[19] Though this risk is very small for adults, it is significantly higher in pediatric and young adult patients.[20][21] Therefore, when the potential benefits of a more precise treatment plan are small, a conventional radiation plan with a lower integral dose may be preferable.
For example, consider a pediatric patient who needs 45 Gy* of radiation to the mediastinum. This dose can certainly be focused from 360 degrees via TomoTherapy, with the 45 Gy isodose line kept precisely around the contoured mediastinum, but the lungs, soft tissues, etc. will receive low-dose radiation (5 Gy or less). In contrast, conventional radiation treatment from two fields, front to back, would limit all radiation to the pathway that encompasses the mediastinum. The anterior and posterior chest would receive higher doses, but the risks to those regions are very small, and there would be no radiation outside of those two fields (in this case laterally). The total absorbed dose, the integral dose, with the conventional two-field technique would be smaller, as would the carcinogenic risk. The mediastinum would receive the same therapeutic dose regardless of method. Since pediatric and young adult cancer patients can have high cure rates and many decades to live, a conventional treatment plan would be preferable in the case described.
Finally, overconfidence in the accuracy of imaging may increase the chance of missing lesions that may be invisible on the planning scans (and therefore not included in the treatment plan) or that move between or during a treatment (for example, due to respiration or inadequate patient immobilization). Lastly, it may not be clinically necessary or cost effective to offer an expensive and resource-consuming treatment to patients who would not benefit from highly conformal radiation treatment.
CONCLUSION
TomoTherapy® Hi•Art® is a highly precise radiotherapeutic delivery system with state of the art image guidance. When highly conformal radiation plans are indicated, it allows radiation oncologists to minimize high doses of radiation to healthy tissues while ensuring coverage of the intended treatment volume. It allows treatment of tumors that might have been considered untreatable in the past due to close proximity of vital organs and structures. It has stimulated research into the development of new radiotherapeutic treatment options with the focus on reducing treatment length and toxicity while increasing local control and survival for patients with cancer.
References
[1] Roentgen WC: Uber eine neue Art Von Strahlen. Sitzgsber Physik Med Ges Wuerzburg 1895;137:132
[2] Hutchinson F: Molecular basis for action of ionizing radiations. Science 1961;134:533,
[3] De Neve W, Claus F, Van Houtte P, et al. Intensity modulated radiotherapy with dynamic multileaf collimator. Technique and clinical experience. Cancer Radiother. 1999;3:378-92
[4] Mackie TR, Holmes T, Swerdloff S, et al. Tomotherapy: a new concept for the delivery of dynamic conformal radiotherapy. Med Phys. 1993;20: 1709-19
[5] Timmerman RD, Papiez L, McGarry R, et al. Extracranial stereotactic radioablation: Results of a phase I study in stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2003;57(2 Suppl):S280-S281
[6] Whyte RI, Crownover R, Murphy MJ, et al. Stereotactic radiosurgery for lung tumors: Preliminary report of a phase I trial. Ann Thorac Surg. 2003;75:1097-1101
[7] Nagata Y, Takayama K, Matsuo Y, et al. Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic bodyframe. Int J Radiat Oncol Biol Phys. 2005;63:1427-1431
[8] Timmerman R, Papiez L, McGarry R, et al. Extracranial stereotactic radioablation: Results of a phase I study in medically inoperable stage I non-small cell lung cancer patients. Chest 2003; 124:1946-1955.
[9] Lai S, Lai H, Krongrad A, et al. Overall and disease-specific survival after radical prostatectomy: geographic uniformity. Urology. 2001; 57:504-509
[10] Zelefsky MJ, Levin EJ, Hunt M, et al. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;15;70(4):1124-1129
[11] Zelefsky MJ, Levin EJ, Hunt M, et al. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;15;70(4):1124-1129
[12] Zelefsky MJ, Chan H, Hunt M, et al. Long-term outcome of high dose intensity modulated radiation therapy for patients with clinically localized prostate cancer. J Urol. 2006;176:1415-1419
[13] Brenner DJ, Martinez AA, Edmundson GK, et al. Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys. 2002;52:6-13
[14] D'Souza WD, Thames HD. Is the alpha/beta ratio for prostate cancer low? Int J Radiat Oncol Biol Phys. 2001;51:1-3
[15] King CR, Fowler JF. A simple analytic derivation suggests that prostate cancer alpha/beta ratio is low. Int J Radiat Oncol Biol Phys. 2001;51:213-214
[16] Kupelian PA, Thakkar W, Khuntia D, et al. Hypofractionated intensity-modulated radiotherapy (70 gy at 2.5gy per fraction) for localized prostate cancer: Long term outcomes. Int J Radiat Oncol Biol Phys 2005; 62:1322–1331
[17] eoh EE, Fraser RJ, McGowan RE, et al. Evidence for efficacy without increased toxicity of hypofractionated radiotherapy for prostate carcinoma: early results of a phase III randomized trial. Int J Radiat ncol Biol Phys. 2003;55:943-955
[18] Lukka H, Hayter C, Julian JA, et al. Randomized trial comparing two fractionation schedules for patients with localized prostate cancer. J Clin Oncol. 2005;23:6132-6138
[20] Kellerer, AM, and Barclay, D. Age dependencies in the modelling of radiation carcinogenesis. Radiat Prot Dosim 1992;41:273-281
[21] Land, CE, Boice JD, Shore, RE, et al. Breast cancer risk from low-dose exposure to ionizing radiation: results from parallel analysis of three exposed populations of women. J Natl Cancer Inst. 1980;65:353-376
Neither Jeff Eshleman, M.D. nor any member of his immediate family have any relevant relationships to disclose with any corporate organizations associated with the manufacture, license, sale, distribution or promotion of a drug or device.
Jeffrey S. Eshleman, M.D.
Lancaster Radiology Associates, Ltd.
2102 Harrisburg Pike
Lancaster, PA 17604
jseshlem@LGHealth.org
* Gray (Gy). The international system unit of radiation dose expressed in terms of absorbed energy per unit mass of tissue. 1 gray = 1 Joule/kilogram and also equals 100 rad.