 Click to Print Adobe PDF
Click to Print Adobe PDF
Winter 2008 - Vol.3, No.4
OPTIONS IN BREAST RECONSTRUCTION SURGERY - A REVIEW
John C. Schantz, M.D.
Plastic Surgery Associates of Lancaster, P.C.
|
 |
ABSTRACT
This article reviews the various options in reconstructive breast surgery following breast cancer, which vary with the different indications for the procedures. The multiple circumstances that can lead to the need for reconstruction are reviewed, the different reconstructive procedures are presented and illustrated, and representative case studies are provided.
INTRODUCTION
Following is a discussion of different options for breast reconstruction. The various approaches are categorized according to the initial surgery that is carried out to control the malignancy, and the regimen of chemotherapy and/or radiation that is recommended afterward. The choices are inevitably influenced by the initial clinical circumstances of the cancer, including its extent and aggressiveness, but in the end the patient must be the final arbiter of the choice of procedure. Each option is demonstrated with an illustrative case history.
PROCEDURES Prophylactic Subcutaneous Mastectomy
Prophylactic subcutaneous mastectomy has become an increasingly popular option for women with an increased risk of developing breast cancer, since it has been documented to reduce that risk by 90 percent or more.1 Although the procedure has always been used selectively, at one time it was viewed by some in the medical community as overly aggressive, even somewhat radical, until more recent data confirmed its efficacy. Currently, prophylactic subcutaneous mastectomy is accepted as a reasonable consideration in patients with conditions that substantially increase the risk of developing breast cancer, including:
- cancer in the opposite breast in a relatively young patient;
- a strong family history of breast cancer;
- presence of the BRCA1 and BRCA2 genes that are associated with breast cancer;
- extensive fibrocystic breast disease that makes it difficult to follow the patient and increases the likelihood that an early malignant mass will not be detected. This could lead to more breast biopsies, with consequent breast scarring and deformities. These problems are magnified by the increased anxiety these patients endure with each subsequent biopsy.
Patients in categories 2–4 would of course be considered candidates for bilateral subcutaneous mastectomies. And, as we increase our understanding of the factors that contribute to the development of breast cancer, other indications will surely be added to the list.
It is important to recognize that subcutaneous mastectomy does not remove all the breast tissue, so the possibility of developing breast cancer still exists, and careful long-term follow-up is necessary. However, since the remaining tissue is superficial, follow-up breast exams can be performed more easily and accurately.
As a general rule, reconstruction can either be performed immediately at the time of subcutaneous mastectomy, or it can be delayed a few weeks to be sure the entire skin envelope is viable.2 A decision must also be made initially whether the nipple itself should be cored out and left intact, or whether it should be removed while the areola is spared.
While several reconstructive options exist, the most popular method involves inserting a silicone or saline implant so that its upper aspect is beneath the pectoralis muscle, and the lower aspect is in the subcutaneous plane.
Illustrative case: A typical case is a 48 year old, BRCA1 positive, nulliparous female with a family history of breast cancer in a maternal grandmother, a maternal aunt, and an older sister. The patient underwent bilateral prophylactic subcutaneous mastectomy 20 years ago with immediate silicone implant reconstruction. She reports normal follow- up exams until last year when she noted one breast became firmer than the other and, upon examination, was found to have developed a Grade 3 capsular contraction which means that the tissue envelope surrounding the implant became tight and can be associated with pain and a change in shape of the breast. After discussion of her options, she elected to change to saline-filled implants, and requested that the opposite side implant be replaced also, because of the age of the implant and for better symmetry. (With saline implants it is easier for the patient to tell if there is a leak or deflation. Also, unlike silicone implants, there is no recommendation for biannual MRI’s to look for silent rupture.) She continues to carry out breast self-examinations monthly, is confident that she is doing good assessments, and her anxiety about developing breast cancer has diminished.
Lumpectomy and Radiation
Another common procedure after the diagnosis of breast cancer is lumpectomy followed by radiation therapy. Whether a significant breast deformity results depends not only upon the amount of breast tissue removed by the lumpectomy in relation to the size of the breast, but also on the response of the breast tissue to the effects of radiation. If the patient desires reconstruction, caution is warranted. When reconstruction is done after radiation therapy, capsular contraction or excessive firmness of the implant occurs very frequently, resulting in a reconstruction that can be painful because of the capsule tightness which results. This is also often accompanied by deformation of the implant shape and a poor aesthetic appearance.3
It is best to allow the radiated tissue to stabilize, as the treated tissue can continue to shrink for a period of time, and the volume of tissue necessary for breast restoration could be underestimated.
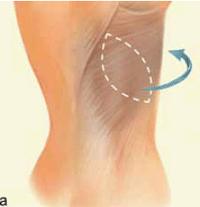
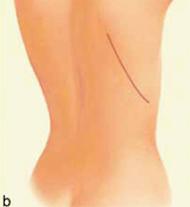
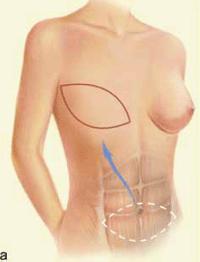
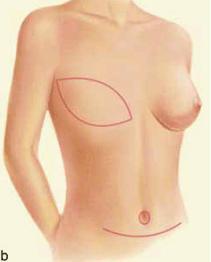
The principle to keep in mind during reconstruction after radiation is that the tissue used for the reconstruction should have its own blood supply; healing is always more challenging in an irradiated field. Therefore, flaps to supply added volume are commonly created from the latissimus dorsi harvested from the lateral midback (Figure 1a, b), or a transverse rectus abdominus myocutaneous flap (TRAM flap) harvested from the abdomen (Figure 2a, b). The use of tissue expanders followed by implants cannot be recommended after radiation because of the high likelihood of developing a significant capsular contraction with breast deformity, excessive firmness and possibly pain as discussed previously.4
Illustrative case: After a lumpectomy and radiation therapy, a 43 year old active, right-handed mother of two, who keeps fit by playing tennis twice a week, decided to have her left breast reconstructed with a left latissimus dorsi flap. Because she wore a size 36B bra, was 5 feet 5 inches tall and weighed 146 pounds, she had adequate volume for a reconstruction without the need for an underlying implant. After six weeks of healing, she was able to resume her tennis having preserved strength in her dominant arm and abdominal muscles.
| Figure 1 (a and b): A latissimus dorsi flap uses muscle, fat and skin from the back tunneled to the mastectomy site and remains attached to its donor site, leaving blood supply intact. |
|
|
|
| Copyright permission was obtained from the American Society of Plastic Surgeons. The American Society of Plastic Surgeons kindly provided these images from their brochure for “Breast Reconstruction” #1810-0907. |
| Figure 2 (a and b): A TRAM flap uses donor muscle, fat and skin from a woman’s abdomen to reconstruct the breast. |
|
|
|
| Copyright permission was obtained from the American Society of Plastic Surgeons. The American Society of Plastic Surgeons kindly provided these images from their brochure for “Breast Reconstruction” #1810-0907. |
Modified Mastectomy with Axillary Sampling
Modified mastectomy with axillary sampling is another procedure that is commonly recommended by the surgical breast oncologist. There are multiple reconstructive choices available, as well as issues of timing of these procedures, because reconstruction can be immediate or delayed. Because an ellipse of skin that includes the biopsy site is frequently incorporated in the mastectomy specimen, reconstruction ordinarily requires additional replacement skin, which may be obtained by using a latissimus dorsi myocutaneous flap, a microvascular free flap (commonly harvested from the abdomen or buttock), or a tissue expander.
Tissue expanders are commonly placed beneath the pectoralis muscle at the time of the mastectomy. They are silicone shell devices which have a magnetized cup and a self-sealing port. After an initial healing period of about two weeks, a magnet is used to identify the port by triangulation, and it is filled weekly with saline. The skin stretches in response to the subsequent saline fills much like the abdomen does in pregnancy. When the desired volume has been reached to match the native opposite breast, the tissue expander is removed and is replaced with a silicone/saline implant through a portion of the original scar. Even if chemotherapy is added to the therapeutic regimen, tissue expansion can proceed. To reduce the likelihood of infection in the permanent implant, the tissue expander is generally left in place through the entire typical six months course of chemotherapy. The expander is generally exchanged for the permanent implant only after the chemotherapy has been completed and the white blood cell count has fully recovered.
When tissue expanders have been inserted at the time of the original mastectomy, and a decision has then been made post-operatively to use radiation therapy, the results of reconstruction have generally been disappointing, as the implant tends to get firm and perhaps even distorted. If the patient has been properly counseled pre-operatively, she will be aware that radiation has these effects on tissue expanders/implants. With this background, she will be able to decide whether to proceed with the expander/implant process even though the final result may be sub-optimal, or to remove the tissue expander and choose one of the reconstructive options that uses autologous tissue with it’s own blood supply. Of course, no filling of the tissue expander can be done while the radiation is being given or the treated breast will not receive the proper dose of radiation. However, one would like to resume the filling of the tissue expander immediately after radiation is completed or the radiation fibrosis will make expansion difficult.
Latissimus dorsi myocutaneous flaps, which often require an underlying silicone/saline implant to achieve adequate volume, can be done as a single stage breast reconstruction. Likewise, both transverse rectus abdominus myocutaneous (TRAM) flaps, and free microvascular abdominal or buttocks flaps, have the advantage of being single-stage reconstructive procedures, but either technique is more technically demanding.
Illustrative case: A 54 year old who is 5 feet 6 inches tall, weighs 138 pounds and wears a size 38 C bra, discovered a mass in her right breast confirmed by mammogram and biopsy. Because of the size of the mass and its cell type, her breast care team recommended modified radical mastectomy with axillary sentinel node excision. She had a preoperative visit with a reconstructive surgeon to review her options, and expressed a preference for a first-stage breast reconstruction using a tissue expander at the time of the mastectomy. This decision was supported by the fact that she was in good general health and did not smoke which is a red fl ag for all breast reconstructions. The nicotine in tobacco products is a vasoconstrictor that reduces blood flow to tissues which require good blood flow to promote healing. (Most reconstructive surgeons will not perform these procedures unless the patient stops smoking without cheating for a minimum of 2–3 weeks preoperatively and abstains for 3 weeks postoperatively. Even so, the success rate is diminished. Of course the patient cannot use one of the smoking cessation aids that contain nicotine products during the period of abstinence as that would defeat the purpose by merely substituting one form of nicotine for another. Smoking also causes more coughing and thereby could increase postoperative discomfort.)
Unfortunately, the sentinel node biopsy revealed micrometastasis and post-operative chemotherapy (4 cycles over 6 months) was carried out. However, tissue expansion with the weekly saline infusions continued until a total fill volume of 550 ml was achieved over the course of eleven visits. Because of some issues with nausea and vomiting from the chemotherapy, the eleven visits, which began two weeks after surgery and would ordinarily have been done weekly thereafter, stretched to 15 weeks post-operatively. Additionally, she still had to finish her chemotherapy and allow her white blood cell count to recover. As a result, her second stage breast reconstruction with removal of the tissue expander and placement of the silicone/saline implant wasn’t accomplished until 30 weeks after her mastectomy.
ADDITIONAL CONSIDERATIONS
Nipple reconstruction: This can be achieved in a variety of ways and is usually a later procedure. Projection of the nipple can be created by using various local flaps, but with all of the methods there is a tendency for some regression of projection to occur over time. A newer, but simpler method of nipple reconstruction is done by means of tattoo. The areola is created by tattooing, after which the nipple can be simulated by using a darker pigment. Beneath the nipple, one of the longer lasting “fillers” can be injected to add projection. Over time, both the tattoo and the filler may need to be refreshed.
The contralateral breast: To gain better symmetry, there may be a need to modify the opposite breast. If the operated breast has been reduced in size, a reduction mammoplasty can be done on the opposite breast to reduce its size comparably and to elevate it. If elevation alone is all that is necessary, a mastopexy may be employed.
Financial considerations: From the standpoint of insurance reimbursement, breast reconstruction following cancer surgery is considered part of the necessary surgical therapy, not cosmetic surgery. Indeed, Pennsylvania law not only requires insurance companies to cover breast reconstruction, but also provides coverage to allow modification of the opposite breast for symmetry.
CONCLUSIONS
A variety of approaches has been presented for addressing the problems that remain after resection of breast cancer. Long-term follow-up is necessary after all of the above techniques for breast reconstruction, not only because of the risk of recurrent cancer, but because there is a possibility that additional revision surgery will be needed. Likewise, patients should be informed that, although these procedures are called breast reconstruction, the resulting breast will never have the same feel, look, or sensation of a natural breast. It is perhaps best thought of as an internal breast prosthesis which, if well done, mimics the appearance of the natural breast.
References
[1]Spear S, et al. Prophylactic Mastectomy and Reconstruction: Clinical Outcomes and Patient Satisfaction. Plastic Reconstructive Surgery 112: Number 1, 1-9, 2008.
[2]Sullivan S, Fletcher D, Isom C, Isik F. True Incidence of All Complications Following Immedicate and Delayed Breast Reconstruction. Plastic and Reconstructive Surgery 112:Number 1, 19-28, 2008.
[3]O'Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: A randomized trial. Lancet 359:2235, 2002.
[4]Heller L, Parker P, et al. Interactive Digital Education Aid in Breast Reconstruction. Plastic and Reconstructive Surgery 122:Number 3, 717- 724, 2008.
Neither Dr. Schantz nor any member of his immediate family have any relevant financial relationships with any corporate organizations associated with the manufacture, license, sale, distribution or promotion of a drug or device to disclose.
John C. Schantz, M.D. Plastic Surgery Associates of Lancaster, P.C. 554 North Duke Street Lancaster, PA 17602 JCSchant@LGHealth.org