|
“A good laugh and a long sleep are the best cures in the doctor's book.”
- Irish Proverb
ABSTRACT
Sleep disorders (SDs) affect millions of Americans, but though their consequences can be devastating, they are often underdiagnosed or misdiagnosed. Knowledge of the diverse symptoms and causes of the different types of SDs is critical to accurate diagnosis and appropriate treatment. SDs are classified into extrinsic, intrinsic, and circadian rhythm types on the basis of their underlying causes. Diagnosis requires a thorough evaluation that begins with the patient’s main complaints, followed by medical history, family history, social history, and physical examination, and then proceeds to selected studies including the Epworth Sleepiness Scale, Polysomnography tests, and Multiple Sleep Latency Test.
INTRODUCTION
Physicians frequently encounter patients with complaints of sleepiness. As far back as 1992, the National Commission on Sleep Disorders Research concluded that approximately 40 million Americans suffered from specific sleep disorders, with an additional 20 to 30 million experiencing intermittent sleep-related problems. The Sleep in America Poll conducted by the national Sleep Foundation in 2005 1,2 reported that as many as 44% of those polled had a sleep problem, about 44% indicated they experience daytime sleepiness 3 days out of 7, 26% had problems with intimate relations because of sleepiness, and 38% complained of waking up un-refreshed at least a few nights per week. In the same poll only 45% indicated they would discuss these issues with a doctor, and 18% expected the problem to go away or would do nothing. The prevalence of sleep disorders is rising, and it is estimated that nearly 80 million Americans will experience a sleep problem by 2010.1
The major consequence of sleep disorders is excessive daytime sleepiness (EDS), which is defined as feeling drowsy and unable to stay awake to accomplish daytime activities. Epidemiologic studies of EDS have been inconsistent because of its various definitions, but it affects 4-16% of the population and there is clear evidence that it is unhealthy.3 The social and economic consequences of EDS are dramatic and substantial. In addition to poor job performance, impaired quality of life, and diminished sense of well-being, many road accidents and some industrial disasters such as those at Chernobyl and Three Mile Island are attributable to excessive sleepiness. EDS associated with sleep disorders can lead to cardiovascular morbidity and even mortality. The National Commission on Sleep Disorders Research estimates that the direct cost of sleep disorders approaches 16 billion dollars annually, and the estimated cost of reduced workplace productivity is 150 billion dollars.1,2
Notwithstanding these data, the public as well as physicians often underestimate the impact of sleepiness, and sleep disorders are often under diagnosed or misdiagnosed. According to the National Sleep Foundation, 70% of American adults who claimed to have sleep problems never discussed these problems with a health care provider. Clearly, in order to diagnose and treat sleep disorders, the physician must not only have a basic understanding of the different sleep disorders, but must be alert to their prevalence.1
NORMAL HUMAN SLEEP
Sleep is a very important homeostatic mechanism, and no human can survive without it for extended periods. The basic sleep drives are both homeostatic and circadian (varying with the 24 hour day/night cycle).4 The stages of sleep were first classified in the 1930’s 5 and REM (rapid eye movement) sleep was defined in the 1950’s.6 There are 4 stages of non-REM sleep which are defined by EEG recordings based on the amplitude and frequency of Alpha and Delta waves. Stages 3 and 4 are often combined into one called “Slow Wave Sleep” (SWS). The other stage, REM sleep, also has a characteristic EEG, and is associated with complete loss of muscle tone except for rapid eye movements that occur in bursts. The brain seems to be active at this point while the body is quiescent, and dreams often appear. REM sleep occurs in 90 minute to 120 minute cycles alternating with non-REM cycles.
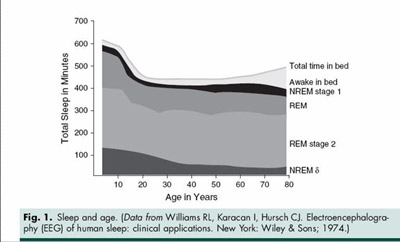
It is thought that each defined stage has some important restorative function: REM sleep for the physiologic brain functions; non-REM sleep for bodily functions. The function of each stage has been studied but for the most part no consensus has been reached about the restorative purpose of each stage. It is clear, however, that loss or fragmentation of any of these stages often results in non-restorative sleep and likely excessive daytime sleepiness. Sleep usually progresses from wakefulness into Stage 1, then to Stage 2, with SWS occurring the first 3rd of the night. One, usually short, REM period occurs during this period. This is usually followed by two or three (90 min – 120 min) cycles of REM interspersed with non-REM sleep (Figure 1). Each REM event seems to be longer as the night progresses. One usually awakens after the last REM period of the sleep cycle.
Normal sleep in a young adult is 90– 95% efficient (i.e. the percentage of time in bed actually sleeping), with about 50% of sleep spent in Stage 2, 20-25% in REM, 1-5% in stage 1, and the remainder in SWS. When sleep is disordered, disrupted, or fragmented, the restful sleep stages are too brief, and sleepiness results.
SLEEP IN AGING PATIENTS
Since aging affects so many physiological processes, it’s no surprise that 50% of older adults complain of poor sleep, which usually means fragmented, light, or restless sleep with frequent awakenings. These changes often compromise quality of life by causing daytime sleepiness.7 In the elderly, homeostatic mechanisms of sleep are thought to weaken, while sensitivity to external stimuli increases, all leading to more fragmented sleep.8,9 Poor sleep in the elderly may result from their higher frequency of sleep disorders such as obstructive sleep apnea 10,11 and restless leg syndrome - OSA and RLS.12 Common medical co-morbidities such as COPD, CHF, BPH, arthritis and other painful conditions also contribute to increasing sleep fragmentation in the elderly. Older adults also have less tolerance for sleep phase shifts and sleep deprivation.
Studies of sleep architecture in the elderly indicate a decrease in total sleep time, sleep efficiency, and SWS. Awakening after the onset of sleep Stages 1 and 2 is more common, and REM sleep can be fragmented even while its duration remains constant.13 Even with these changes there are many healthy older adults who do not suffer untoward side effects from less sleep.
SLEEP DISORERS ASSOCIATED WITH EXCESSIVE DAYTIME SLEEPINESS
On the basis of the underlying causes, we classify sleep disorders into extrinsic sleep disorders, intrinsic sleep disorders, and circadian rhythm disorders, but all three shares a common thread of excessive daytime or inappropriate sleepiness.
Extrinsic Sleep Disorders
As the name implies, extrinsic sleep disorders are due to external or environmental disturbances, including temperature, noise, light, heavy meals, drugs, alcohol, caffeine, and erratic sleep times. They are relatively easy to diagnose and treat, often by simply practicing “sleep hygiene:” avoiding coffee, alcohol, heavy meals, and physical activity before going to bed; setting a good sleep routine; and making the sleep environment suitable for sleep in terms of light, temperature, and noise. 1,2,14
Intrinsic Sleep Disorders
Common intrinsic sleep disorders include obstructive sleep apnea (OSA), chronic sleep deprivation (CSD), narcolepsy, and restless leg syndrome (RLS); all are more difficult to diagnose and treat.
Obstructive Sleep Apnea
OSA is characterized by repetitive airflow reduction caused by collapse of the upper airway during sleep. It afflicts almost 20 million Americans: 4% of middle-aged men and 2% of middle-aged women.15 It is one of the commonest causes of EDS, and is more prevalent in adults than asthma. It has also recently been associated with hypertension, coronary artery disease, congestive heart failure, and stroke.
The most common symptoms include EDS, snoring, and apnea (cessation of airflow that lasts at least 10 seconds). In the normal waking state the tendency of the upper airway to collapse with each negative pressure inspiration is balanced by the outward forces of the pharyngeal dilator muscles driven by the central and possibly autonomic nervous system.16 In patients with OSA, especially while sleeping, the natural tendency to keep the airway patent is overwhelmed because of obesity, anatomical characteristics of the airway components such as the soft palate, tongue, tonsils, and mandible, and possibly abnormal contraction of the pharyngeal muscle dilator as a result of altered central control.17
There are numerous pathophysiologic effects of repetitive collapse of the upper airway. The increased respiratory effort often associated with snoring at the end of the apneic interval causes the release of catecholamines, which has also been attributed to the hypoxia that occurs with each episode. Both phenomena probably play a role but it is uncertain which predominates. The effect of catecholamines on the autonomic nervous system is likely a major factor in the development of hypertension and the cardiovascular complications found in OSA patients.
The most important risk factors for OSA include obesity, fat accumulation around the neck, gender, family history, and hypertension. The consequences of OSA include decreased cognitive performance, impaired quality of life, mood disturbances, impaired vigilance, and higher risk of automobile accidents.18 All of these factors illustrate the importance of early recognition and treatment.
Treatment options include continuous positive airway pressure (CPAP); surgical interventions such as (uvulopalatopharyngoplasty); weight loss; treatment of nasal allergies; and positional therapies. The latter conservative treatments are usually more effective for mild rather than severe OSA, and should be used as adjuncts to CPAP and surgical intervention. 19
Chronic Sleep Deprivation
Chronic sleep deprivation may be the result of sleep that is fragmented, rather than merely too brief. Its direct consequence is EDS, with its usual problems of poor concentration at work, diminished sense of well-being, and increased accidents. Since stress and other sleep disorders are the main risk factors for sleep deprivation, the increased demands of work and social activities in industrialized countries has led to a rising incidence of sleep deprivation.
Patients can minimize these causative factors by keeping a regular sleep schedule, increasing the time allotted for sleep, practicing sleep hygiene, such as controlling environmental cues including radio, TV, light, and temperature, and avoiding distracting bedtime activities such as eating or balancing check books. “Sleep hygiene” means creating the best possible sleep-promoting environment.2
Narcolepsy
Narcolepsy is a rare but dramatic neurological sleep disorder that affects 1 in 2000 Americans.20 It is characterized by the brain’s inability to regulate the sleep-awake cycle, and it can be considered either a disease of REM sleep or an intrusion of REM sleep into the wake state.21 In addition to EDS, symptoms include sudden weakness or loss of muscle tone without loss of consciousness (cataplexy*), hallucinations at the onset of sleep or upon awakening, sleep paralysis, and sleep fragmentation.
Many, but not all, cases of Narcolepsy cluster in families, but the basic pathophysiology involves an autoimmune process that destroys the cells in the hypothalamus that produces a neuropeptide called hypocretin (orexin). Cataplectic narcolepsy seems more closely associated with hypocretin deficiency than are any of the other symptoms of narcolepsy.22 HLA marker (DQB1*0602) has been associated with cataplectic narcolepsy, and there seems to be a tight association between the HLA marker and hypocretin.23 A study of narcolepsy subjects with cataplexy who were either positive or negative for this marker showed no IgG reactive autoimmune connection to hypocretin 1 or 2.24 Thus, the immune connection between HLA marker DQB1*0602 and hypocretin has not been completely worked out. Since the autoimmune process usually occurs in adolescence, most patients present as young adults.
Unfortunately, though the pathogenesis is now understood, cure is a long way off. Treatment options for narcolepsy include both lifestyle modifications and medications. The former involves avoiding nicotine and alcohol, while the most commonly used medications include modafanil for the treatment of EDS and gamma-hydroxybutyrate (GHB) for the treatment of cataplexy.14,19,20 Recent mammalian studies have shown that hypocretin has many wide projections in the brain that are closely linked to dopaminergic, noradrenergic and serotinergic pathways, and are mostly excitatory. Stimulant drugs such as amphetamines work on the monoaminergic pathways.
Modafanil is believed to work selectively through the sleep-wake centers to activate the cerebral cortex, which is essential for wakefulness. The mechanism of action of GHB is for the most part unknown.
Restless Leg Syndrome (RLS)
RLS is characterized by abnormal sensations of creeping or crawling in the legs and an uncontrollable urge to move the legs during sleep. As many as 12 million Americans are affected, with women slightly more common than men. Stress and alcohol can exacerbate the symptoms. More than 80 percent of affected patients also experience periodic limb movement disorder (PLMD), characterized by involuntary leg twitching or jerking during sleep. Conversely, most people with PLMD do not experience RLS.
The pathophysiology of RLS is related to ineffective dopamine transport in the brain due to defects in iron metabolism in the substantia nigra and putamen. Thus, certain reversible forms of RLS can be treated by iron replacement. In about one third of patients, a strong family history suggests a genetic component. A major genetic locus on chromosome 12q has been identified in the familial phenotype.12
Treatment options include dopamine receptor agonists such as ropinirole, which is thought to work centrally in the areas of ineffective dopamine transport. Non-pharmacological management includes physical activities, rubbing the leg, and taking a warm bath before bedtime.3
CIRCADIAN RHYTHM DISORDERS
Misalignment of the normal circadian day/night cycle is the primary cause of circadian rhythm disorders. The pathophysiology is for the most part unknown, but the supra-chiasmatic nucleus (SCN), located behind the optic chiasm near the hypothalamus, is felt to play a key role, because it is responsible for the 24 hour circadian cycles of the body. Common circadian rhythm disorders include delayed sleep phase syndrome (DSPS), advanced sleep phase syndrome (ASPS), and shift work sleep disorder (SWSD).
Delayed Sleep Phase Syndrome (DSPS)
DSPS is characterized by a delayed sleep-wake cycle. It generally occurs in young people, who report that the onset of sleep and the time of waking up are intractably later than desired. Once asleep, they generally sleep well, and results of all-night Polysomnography tests (see below) are essentially normal except for the delayed onset of sleep. Treatments include attempting to reset the circadian rhythm by exposure to bright lights in the early morning and avoidance of bright lights in the late evening.19
Advanced Sleep Phase Syndrome (ASPS)
In ASPS the circadian cycle is advanced, so that sleep occurs too early. It affects mostly older people with daytime sleepiness and an inability to stay awake until the desired bedtime. Treatment for ASPS also involves resetting the circadian rhythm with exposure to bright light in the early morning hours. In addition, many patients with ASPS benefit from wearing sunglasses when outside in bright summer mornings.19
Shift Work Sleep Disorder (SWSD)
SWSD affects millions of Americans who work at night. It causes excessive sleepiness at night, and insomnia in the daytime even when there are opportunities to sleep. Other symptoms include poor concentration, headache, and fatigue, with reduced productivity and increased accidents in the workplace. Treatment can be extremely difficult. Modafinil is the only medication approved for excessive sleepiness associated with SWSD.19
EVALUATION OF THE PATIENT
The approach to patients with sleep disorders requires persistent and patient evaluation with a focus on the patient’s main complaints. In addition, evaluation should include the patient’s sleep pattern and sleep habits as well as that of their bed partner. The family history, social history, and medication list also provide important information.
History
Since many patients with sleep disorders are not aware of their symptoms, or deny their problems for various reasons, it is important to confer with the patient’s spouse or bed partner. It is also important to obtain a brief profile that includes the patient’s age, sex, occupational status, marital status, and living arrangements. Abuse of alcohol, caffeine, nicotine, and illicit drugs can markedly affect sleep patterns.14
A wide spectrum of diseases including seizure disorders, Parkinson’s disease, asthma, and almost all painful illness can cause significant sleep disturbance. In addition, patients with depression and anxiety often complain of EDS. It is, therefore, important to rule out any other problems when diagnosing a sleep disorder.14 A variety of medications can alter sleep and wakefulness. Diuretics have an indirect effect on sleep, while theophylline and other bronchodilators can affect sleep directly. Herbal supplements and illicit drugs also have a variety of sedating and stimulating effects, but patients often omit mentioning them.
Since both narcolepsy and OSA have a genetic basis, a positive family history of either one increases the likelihood of developing these disorders.14
Physical examination
Since sleep disorders are often associated with other physical problems, such as obesity and heart failure in patients with severe OSA, the physical examination should not only check weight, blood pressure, and the heart and lungs, but also the patient’s breathing rate, neck circumference, naso- and oropharyngeal dimensions, tongue dimension, and soft palate.
DIAGNOSIS
Although not all people with EDS require medical tests, physicians should order diagnostic tests if the EDS affects a patient’s quality of life, medical condition, or safety. Diagnostic evaluation of sleepiness generally includes Epworth Sleepiness Scale, Polysomnography, and Multiple Sleep Latency Test.
Epworth sleepiness scale (ESS)
ESS is a self-administered sleep questionnaire widely used to predict daytime sleepiness. Patients are asked to rate from 0 to 3 their tendency to doze in 8 hypothetical situations, including sitting and reading; watching television; sitting inactive in a public place; as a passenger in a car for an hour without a break; lying down to rest in the afternoon when circumstances permit; sitting and talking to someone; sitting quietly after a lunch without alcohol; in a car while stopped for a few minutes in traffic. A score of 0 refers to no self-reported sleepiness, whereas a score of 24 suggests the greatest tendency to doze during common daytime situations. A score of 12 or greater is generally considered consistent with a pathologic degree of self-reported daytime sleepiness. 2,14
Polysomnography (PSG)
PSG is the gold-standard for diagnosing OSA. It is a laboratory-based, technician-attended recording of sleep architecture, airflow, and cardiac rhythm, through a variety of measurement techniques including electroencephalography, electro-oculography, electromyography, nasal pressure detector, strain gauge, pulse oximetry, and electrocardiography. An initial negative study may not rule out OSA, and a second night’s test is often required if clinical suspicion is strong.11
Multiple sleep latency test (MSLT)
MSLT is generally used in conjunction with a PSG the night before, and is done to further quantify EDS and to screen for narcolepsy. It consists of a series of naps (4 to 6), each followed by a 2-hour break. Recordings during the test include EEGs, left and right eye electro-oculograms, and a submentalis electromyogram. A mean sleep latency of five minutes or less is considered abnormal. The number of episodes of REM sleep is also determined, and 2 or more episodes are highly suggestive of narcolepsy.14
THE SLEEP CENTER AT LANCASTER GENERAL HOSPITAL
Lancaster General Hospital has a very active 8 bed laboratory and has recently added beds at its suburban Kissel Hill facility. In the past year alone, approximately 2600 patients were referred for polysomnography, of which about 2200 were for Obstructive Sleep Apnea (85%). Other common conditions seen in the sleep center are Insomnia (2.04%), Narcolepsy (1.47%), and Periodic Limb Movement Disorder with or without Restless Leg Syndrome/ (8.41%).
SUMMARY
Sleepiness is common, and knowledge of sleep disorders is essential to correctly diagnose and appropriately manage such patients. Since sleep disorders are often ignored by the public, it is the physicians’ responsibility to educate patients and to know when to refer them to sleep centers for further study and management.
REFERENCES
1) Ting L, Malhotra A. Disorders of Sleep: An Overview. Prim Care Clin Office Pract 2005; 32: 305-18.
2) Malik S, Kaplan J. Sleep Deprivation. Prim Care Clin Office Pract 2005; 32: 475-90.
3) Ohayon MM. Epidemiology of Excessive Daytime Somnolence. Sleep Medicine Clinics 2006;1(1):1662-1670
4) Hirshkowitz M. Normal Human Sleep: an overview. Medical Clinics of North America 2004; 88(3): 14-26
5) Loomis AL, Harvey N, Hobart GA. Cerebral states during sleep, as studied by human brain potentials. J Exp Psychol 1937; 21:127-44
6) Jouvet M, Michel F, Courjon J. Sur un stade d’activite electrique cerebrale rapide au cours du sommeil physiologique. C R Soc Biol (Paris)1959; 153:1024-28
7) Vitiello MV. Sleep in Normal Aging. Sleep Medicine Clinics 2006 Vol.1(2):1-7
8) Dijk DJ, Duffy JF, Czeisler CA. Age related increase in awakenings: impaired consolidation of non REM sleep at all circadian phases. Sleep 2001; 24: 565-577. (Abstract)
9) Zeplin H, McDonald CS, Zammit GK. Effects of age on auditory awakening thresholds. J Gerontol 1984; 39: 294-300 (Abstract)
10) Wills MH, Perioperative Protection of Patients With Obstructive Sleep Apnea, JLGH 2007; 2: 136-139
11) Olsen, E., Park, J.G., Morgenthaler, T.I. Obstructive Sleep Apnea-hypopnea Syndrome. Prim Care Clin Office Pract 2005; 32:329-59.
12) Desauteis A, Turecki O, Montplaisir J, Sequeria A, Verner A, Rouleau GA. Identification of a susceptibility locus for restless legs syndrome on 12q. Am J Hum Genet 2001; 69(6): 1266-70.
13) Ohayon MM, Carskadon MA, Guilleminault C, et al: Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human life span. Sleep 2004; 27: 1255-1273.
14) Kryger, MH, Thomas, R, Dement, WC ed. Principles and Practice of Sleep Medicine 4th ed., Philadelphia, PA. Elsevier, 2005.
15) Young, T, Palta, M, Dempsey, J, Skatrud, J, Weber, S, Badr, S. The Occurrence of Sleep-disordered Breathing among Middle-aged Adults. N Engl J Med 1993; 328: 1230-5.
16) Remmers JE, deGroot WJ, Sauland EK, et al. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol 1978; 44(6): 931-8
17) Brouillette RT, Thach BT. A neuromuscular mechanism maintaining extrathoracic airway patency. J Appl Physiol 1979; 46(4): 772 -9
18) Engleman HM, Douglas NJ. Sleep 4:sleepiness,cognitive function, and quality of life in obstructive sleep apnoe/hypopnea syndrome. Thorax 2004; 59(7):618-22
19) Kasper, DL., Braunwald, E., Fauci, A., Hauser, S., Longo, D., James, J.L. ed. Harrison's Principles of Internal Medicine 16th ed. New York, NY: McGraw-Hill Companies; 2005
20) Wise, M. Narcolepsy and Other Disorders of Excessive Sleepiness. Med Clin N Am 2004; 88:597-610.
21) Nishino S, Narcolepsy. Sleep Medicine Clinics 2006; Vol 1(1): 62-78
22) Mignot E, Lammers GJ, Ripley B, et al: The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol 2002; 59: 1553-1562. (Abstract)
23) Krahn LE, Pankratz VS, Oliver L, et al: Hypocretin (orexin) levels in cerebro-spinal fluid of patients with narcolepsy: relationship to cataplexy and HLA DQB1*0602 status. Sleep 2002; 25 733-736. (Abstract)
24) BlackIII JL, Silber MH, Krahn LE, et al. Studies of humoral immunity to preprohypocretin in human leucocyte antigen DQB1*0602-positive narcoleptic subjects with cataplexy. Biol Psychiatry 2005; 58: 504-509.
Gregory J. Rossini, M.D.
Medical Director of the Sleep Lab
Pulmonary Associates of Lancaster
555 North Duke Street
Lancaster, PA 17604
717-544-4930
* This term should not be confused with catalepsy, which comes from a Greek root that means a seizure.
|