Spring 2025 - Vol. 20, No. 1
PEARLS OF EDUCATION
Guiding Graceful Aging of the Older Adult
Hehidy Paulino, MD
Family Physician
Family Medicine Residency Program
Penn Medicine Lancaster General Health
The following are pearls from a recent continuing education event at Penn Medicine Lancaster General Health. “Guiding Graceful Aging of the Older Adult” included a variety of presentations, with evidenced-based, multidisciplinary recommendations and referral resources to engage patients and their family members as partners in their care.
This event was sponsored by the Kenneth and Pamela Brubaker Center for Geriatric Learning Endowment and cosponsored by the Philadelphia College of Osteopathic Medicine. These pearls are offered here to increase the reach of this in-person training, published with the intention of helping our community of clinicians better care for our patients.
DEPRESCRIBING FOR THE GERIATRIC PATIENT
Polypharmacy, defined as the use of five or more medications, often leads to redundant prescriptions with inappropriate or no indications, increasing the risk of adverse effects such as falls, disability, or death. Common side effects of patients who experience polypharmacy are sleeping issues, anxiety, depression, weakness, and dizziness. These complaints often prompt a prescribing cascade, additional medication being prescribed to treat side effects caused by a preceding medication, further contributing to the problem.
Deprescribing is a planned and supervised approach to dose reduction or discontinuation of potentially harmful medications. Tools like the American Geriatric Society (AGS) Beers Criteria
® provide guidance regarding potentially inappropriate medications in older adults and advise that caution be exercised with medications that can cause sedation and increase bleeding, as well as with anticholinergics.
1 Guidance for deprescribing is also available from resources such as
Deprescribing.org.
Deprescribing doesn’t mean entirely eliminating medications, but reducing to efficiently optimize symptom management. When deprescribing, it is advisable to focus on reducing one medication regimen at a time, involve specialists in discussion as needed, and consider the specific adverse effects to be addressed, such as sedation.
Utilizing every patient visit to review medication lists and setting clear expectations about the deprescribing process can help patients feel more comfortable with medication changes.
CHRONIC KIDNEY DISEASE IN THE GERIATRIC PATIENT
Physiological decline in renal function typically begins in the fourth decade of life but almost never progresses to end-stage renal disease. With age come significant gross changes in the kidneys, including a 30% reduction in size by the eighth decade and a loss of renal mass by 300 grams by the ninth decade, often accompanied by renal parenchymal cysts.
Estimates of glomerular filtration rate (GFR) have evolved over time, from the Cockcroft-Gault equation in 1976 to more recent equations like the chronic kidney disease epidemiology (CKD-EPI) calculator, which does not include race as a factor. Creatinine-based GFR calculations, while common, may not accurately reflect muscle mass variability among patients, making cystatin C a preferred marker for GFR estimation, particularly when confirmation is necessary.
2
Unique causes of kidney disease in older adults include medication-related issues, volume depletion, sepsis, acute cardiovascular events, systemic illness, and obstructive uropathy. Management in this population requires careful consideration of medication adjustments, with certain medications like renin-angiotensin system blockers, nonsteroidal anti-inflammatory drugs, sodium-glucose cotransporter 2 inhibitors, metformin, and diuretics often needing to be held during sick days due to the increased risk of adverse events. Elderly individuals are more susceptible to adverse drug events at lower doses than younger adults, necessitating renal dosing adjustments.
The initial workup for older adults with suspected kidney disease typically includes assessing GFR, electrolytes, and the albumin-to-creatinine ratio. Risk assessment tools such as the Kidney Failure Risk Index, available on platforms like MD Calc, can aid in determining the need for nephrology consultation, particularly in cases of advanced CKD and during abrupt declines in GFR.
CAPACITY EVALUATION: WHAT, WHY, HOW
The University of Toronto’s Aid to Capacity Evaluation (ACE), when used in the inpatient setting, serves as a valuable resource for assessing the capacity to make informed decisions about health and treatment options. Capacity, in this context, refers to the ability to comprehend relevant information about illness and proposed treatments, and to make decisions that align with one’s own values and preferences.
Determining capacity is crucial for various legal matters, as outlined by Pennsylvania laws, including making financial decisions, giving medical treatment consent, selecting a level of care, appointing an agent, executing living wills and testamentary wills, driving, amending prior documents, understanding prognosis, exercising voting rights, and making end-of-life decisions.
However, capacity evaluations often involve balancing competing interests, such as financial and legal responsibilities, patient and family obligations, and medical considerations. It’s essential to differentiate between choice as opposed to assent, and to discern whether there is understanding and appreciation.
Importantly, when patients demonstrate capacity, they retain the right to make decisions, even if they are perceived as unwise.
ACE offers a structured approach to assessing individuals’ decision-making abilities, ensuring that their autonomy and rights are respected within the bounds of the law and ethical considerations. It includes questions to assess the patient’s understanding of their medical diagnosis, the proposed treatment and alternatives, and the consequences of accepting or not accepting these treatment options, as well as specific questions about autonomy and mental health (see “ACE Sample Questions” below). It is nonproprietary and can be accessed online; practicing with it before use is encouraged.
3
ACE Sample Questions
The Aid to Capacity Evaluation (ACE) questionnaire involves asking a series of seven questions in a conversational style. The interviewer should first have addressed barriers to communication, including vision and hearing problems as well as language barriers.
The interviewer should continuously disclose information about the disease and treatment options to assess understanding, using the patient’s own words if possible to describe disease and treatment. Scoring is not based on whether the interviewer agrees with the patient; this can be challenging, so interviewers should practice ahead of time.
1. What medical problem do you have?
2. What treatment options are there to help you?
3. What other options do you have?
4. Can you refuse or can we stop using the proposed treatment?
5. What could happen if you accept this treatment?
6. What could happen to you if you don’t accept this treatment?
7. If the person’s decision is affected by depression or psychosis: Can you help me
understand why you have decided to accept or refuse treatment? Do you deserve this?
DEMENTIA ASSESSMENT AND MANAGEMENT FOR THE PRIMARY CARE PROVIDER
By 2050, the U.S. population is projected to include more than 89 million individuals aged 65 years and older. While aging brings wisdom and accumulated knowledge, it can also be accompanied by declines in cognitive processing speed, multitasking ability, reasoning, abstraction, and word-retrieval efficiency. Despite these changes, functional abilities are generally preserved, even if patients may find tasks more challenging.
Dementia, characterized by cognitive or behavioral symptoms in multiple domains, poses a substantial challenge to aging populations. The differential diagnosis of cognitive changes includes various types of dementia, including Alzheimer’s disease, vascular dementia, dementia with Lewy bodies, and Parkinson’s disease-associated dementia.
Early evaluation of memory concerns involves a comprehensive assessment. Obtain a history from both patient and informant — including medical history, functional status, medication review, and a review of psychological symptoms — and perform a physical examination and cognitive testing. Initial workup typically includes laboratory tests such as a thyroid hormone level, vitamin B
12 level, comprehensive metabolic panel, and complete blood count, plus assessments for other nutritional deficiencies, urinalysis, and neuroimaging for select cases.
Management of dementia includes optimizing contributing factors and comorbidities, disclosing the diagnosis, coordinating caregiver support, managing behavioral and psychological symptoms, and conducting advance-care planning.
Pharmacological options include cholinesterase inhibitors such as donepezil, rivastigmine, galantamine, and N-methyl-D-aspartate receptor antagonists like memantine. More recently, monoclonal antibodies have proven useful. The use of psychoactive medications like antidepressants and antipsychotics requires careful consideration, particularly in advanced stages of dementia.
Resources such as Penn Medicine LGHP Alzheimer’s and Memory Care, LGHP Geriatrics, and the Lancaster County Office of Aging play a vital role in providing comprehensive care for individuals with dementia.
EMERGING INSIGHTS INTO THE GENETIC BASIS OF ALZHEIMER’S DISEASE
Alzheimer’s disease (AD), the most common cause of dementia globally, is characterized by cortical atrophy, amyloid and tau protein deposition, and insidious cognitive decline, primarily affecting episodic memory and language. Biomarkers such as CSF tau and CSF amyloid, as well as changes in hippocampal volume, may begin to show up 8-15 years before a clinical diagnosis of the disease.
Recent advances in AD treatment include monoclonal antibodies like aducanumab and lecanemab-irmb, which target amyloid deposition. These have been shown to slow cognitive decline, although eligibility criteria include eliminating the possibility of vascular dementia; potential side effects include an increased risk for brain bleeding and swelling.
Familial AD may be caused by a single genetic mutation; non-familial AD is more likely due to multiple factors, a combination of genetic and environmental contributors. Understanding the genetic underpinnings of Alzheimer’s disease, and the interplay of lifestyle and society, aids in risk assessment and personalized management strategies.
Those with one first-degree relative with AD have an increased risk of developing the disorder (relative risk = 1.73), while those with two or more first-degree relatives with AD have an even greater chance (relative risk = 3.98). Genetic testing, particularly for the apolipoprotein E gene, is helpful when preparing to offer lecanemab, but should be accompanied by genetic counseling.
Despite advancements in treatment and understanding of the disease process, dementia remains a significant public health concern, necessitating a multidisciplinary approach including medical, social, and lifestyle interventions to optimize patient outcomes. Clinicians’ focus should remain on maximizing the underlying health status and encouraging a healthy lifestyle, including good diet and exercise.
WOUND CARE BASICS
To effectively address and prevent the recurrence of various types of wounds, clinicians must understand their underlying causes and employ appropriate treatment strategies.
When treating, ensuring a moist wound environment facilitates fibroblast migration to the wound edges, which promotes healing. Biofilm, characterized by a thick yellow appearance and a “wax paper sheen,” prolongs the inflammatory phase of wound healing, thus should be debrided surgically or with medical options like collagenase, such as SANTYL™.
- Venous Stasis Ulcers: Lifestyle modifications can help prevent and manage venous stasis ulcers (see Fig. 1), so patients must be counselled about the value of reducing sodium, walking regularly, achieving a healthy weight, and elevating the legs during rest. Compression stockings, like an Unna boot, are essential for both treatment and recurrence prevention, but caution is necessary in patients with peripheral artery disease.
- Diabetic Ulcers: Management of diabetic ulcers involves addressing various factors. Pressure on the wound itself must be minimized, but compression of the limb may aid in healing. Efforts should be made to appropriately manage sensory neuropathy, impaired immunity, and nutrition. Smoking, obesity, improper footwear, and lifestyle risks must be addressed. Treatment modalities may include debridement, as well as total contact casts, diabetic air casts, and long-term proper footwear. Podiatry evaluations should be performed at each primary care visit, and patients should maintain foot hygiene and moisturization.
- Pressure Injuries: Prevention and treatment of pressure injuries involve relieving pressure on bony prominences and ensuring mobilization. Special attention should be paid to pressure relief and changing the patient’s lying surface to prevent location ulcers, such as the “recliner bottom.”
- Sitting Ulcers: Utilizing the Braden Scale for predicting pressure sore risk can guide preventive measures and treatment. For stage 1-3 ulcers, zinc-oxide barriers, moisturizers, and cocoa butter can aid healing, while stages 3-4 may require enzymatic debriding agents like SANTYL™ or specialized wound dressings such as Hydrofera Blue®, Ag foam silicone, or Iodosorb™.
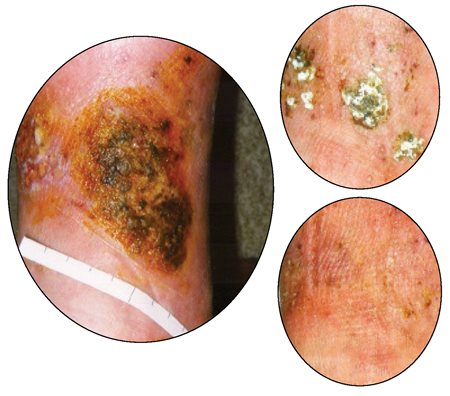
Fig. 1. The healing process of a venous stasis ulcer of the lower leg: above left, at initial presentation; top right, after 3½ months of therapy; bottom right, after 4½ months of therapy. Photos by Prof. Dr. med. Gerd Hoffmann, CC BY-SA 3.0 DE, via Wikimedia Commons.
In addition to these treatments, ensuring adequate intake of nutrients such as zinc, vitamin C, and protein is essential for collagen support and overall wound healing. By addressing the root causes of wounds and implementing appropriate management strategies, health care providers can effectively treat existing wounds and reduce the risk of recurrence, ultimately improving patient outcomes.
PREVENTATIVE SCREENINGS FOR ADULTS AGED 65+
There is value in screening for many cardiovascular and metabolic conditions, including hypertension, hyperlipidemia, abdominal aortic aneurysm, diabetes, obesity, mental health and substance use disorders, as well as osteoporosis, hepatitis, and vision and hearing problems.
The primary goal of cancer screening is to prevent death from cancer by detecting it at an early, treatable stage. The U.S. Preventive Services Task Force recommends regular colorectal and breast cancer screening for eligible individuals, recognizing that the benefits may take more than 10 years to manifest. Tools like the ePrognosis website at
eprognosis.ucsf.edu and the Lee Schonberg Index aid in estimating prognosis and informing decisions about cancer screening.
Clinicians should be able to describe the risks and benefits of trying to detect asymptomatic cancer. In general, it is only necessary if 1) the patient would have developed symptoms during their lifetime, and 2) earlier treatment will reduce morbidity and/or mortality.
In the United States, we have created what may be called “a screening paradox,” meaning that healthy older patients are often under-screened, while those in poor health are often over-screened. Deciding when to stop screening for cancer and other conditions means being able to compare life expectancy with lag time to benefit.
Based on average life expectancy, the benefits are outweighed for breast cancer when a woman reaches 74 years, for colon cancer when a person reaches 75 years, and for prostate cancer when a man reaches 69 years. Remember, though, that no patient is average, so comorbid conditions or exceptionally good health can make stopping screening appropriate at younger or older ages.
Many patients are receptive to discontinuing cancer screening if advised by a trusted physician. However, it is common for patients to underestimate their life expectancy and the potential benefits of screening. Using the right language is crucial, so consider emphasizing that a test may not help a patient live longer rather than suggesting they may not live long enough to benefit.
Having open and honest communication, along with shared decision-making, can empower patients to make informed choices about screening options.
REFERENCES
1. By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria
® for potentially inappropriate medication use in older adults.
J Am Geriatr Soc. 2023;71(7):2052-2081.
2. Benoit SW, Ciccia EA, Devarajan P. Cystatin C as a biomarker of chronic kidney disease: latest developments.
Expert Rev Mol Diagn. 2020;20(10):1019-1026.
3. Aid to Capacity Evaluation (ACE). University of Toronto Joint Centre for Bioethics. Accessed January 24, 2025.
https://www.cmpa-acpm.ca/static-assets/pdf/education-and-events/resident-symposium/aid_to_capacity_evaluation-e.pdf