Spring 2025 - Vol. 20, No. 1
SCIENTIFIC REPORT
Identifying, Preventing, and Treating
Opioid-Induced Constipation


Lawler Patterson
Isabelle Lawler, PharmD
Clinical Pharmacist, Population Health
Lehigh Valley Health Network
Michelle Link Patterson, PharmD, BCACP
Ambulatory Pharmacist Clinician
Penn Medicine Lancaster General Health
INTRODUCTION
Opioid-induced constipation (OIC) occurs in approximately 50% of patients receiving opioid therapy and is somewhat independent of opioid dose, route, or length of treatment.1,2 Patients are at an increased risk of developing OIC if they are elderly, female, or unemployed, and if they have pain and immobility with injury, metabolic abnormalities, or bowel obstructions.2,3 OIC may present immediately after the initiation of opioids or can develop over time with prolonged use.
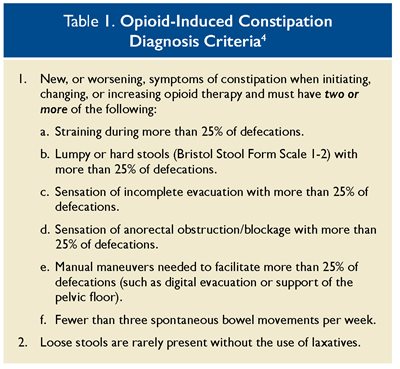
Patients may report straining and/or incomplete emptying, as well as nausea, vomiting, bloating, and abdominal pain. OIC may also present with alternating episodes of diarrhea and constipation. The Rome IV diagnostic criteria (see Table 1) can be used to diagnose OIC, and the Bristol Stool Scale can be used to classify the form of feces. Imaging is not warranted for OIC unless a patient presents with alarm symptoms, such as weight loss, positive fecal occult blood tests, iron deficiency, or a family history of colorectal cancer.3 Patients taking opioids should be regularly screened for the signs and symptoms of OIC.
Opioids cause OIC by activating mu-opioid receptors outside the central nervous system.5 This peripheral activation leads to inhibition of gastric emptying and peristalsis in the gastrointestinal tract. As a result, there is increased absorption of fluids and delayed absorption of medications, ultimately leading to hardened stools. In addition, opioids impact the defecation reflex through increased anal sphincter tone while diminishing rectal sensations.3,6
Patients with medication-induced constipation should be encouraged to stay hydrated by consuming one-and-one-half to two liters of water per day, incorporate soluble fiber in their diet (see Fig. 1), and schedule toileting.2,3,7 When increasing fiber intake or adding fiber supplements, no more than 25-30 grams should be consumed per day, as this may lead to a bloated sensation.3 In addition, patients should be encouraged to exercise regularly, as these lifestyle modifications encourage bowel motility.

Fig. 1. Foods high in fiber.8
Background photo from Formulate Health via Flickr, licensed under CC BY 2.0.
LAXATIVES
At the initial prescription of opioids, patients should be prescribed laxatives to prevent OIC. All types of laxatives, except bulk-forming laxatives, can be used as initial therapy.3 According to the guidelines of the American Gastroenterological Association (AGA) Institute, first-line agents to prevent and treat OIC are traditional laxatives.9 Stimulant laxatives, including senna and bisacodyl, are commonly used and can be purchased over-the-counter. Docusate, a stool softener, can be used in combination with stimulant laxatives and is available as a combination tablet with senna.
Additionally, patients can take osmotic laxatives daily to aid in the treatment and prevention of OIC. The osmotic laxative polyethylene glycol is available over-the-counter, whereas lactulose requires a prescription. Saline laxatives, such as magnesium citrate, are fast acting and may be beneficial in OIC as they provide quick relief in 30 to 180 minutes.3
Magnesium citrate can cause electrolyte abnormalities, volume overload, and elevated sodium, phosphate, or magnesium if a patient has cardiac or renal dysfunction.10 Bulk-forming laxatives should be avoided because they can distend the colon and increase peristalsis, and they can result in worsening abdominal pain and bowel obstruction.3
PERIPHERALLY ACTING MU-OPIOID RECEPTOR ANTAGONISTS (PAMORAs)
In laxative-refractory cases of OIC, the use of more direct-acting agents may be warranted. PAMORAs antagonize peripheral mu-opioid receptors, thereby helping to reverse OIC.3,11 They are less likely to cross the blood-brain barrier because they are less hydrophobic than naltrexone, but patients should be monitored for symptoms of opioid withdrawal such as anxiety, chills, and hyperhidrosis while taking these medications.1,3,11 There are three PAMORAs approved for use in OIC: methylnaltrexone, naloxegol, and naldemedine. In addition, alvimopan, which is currently approved for postoperative ileus, can be used off-label for OIC.9
The AGA Institute guidelines recommend the use of a PAMORA for OIC rather than no treatment.12 When considering the initiation of a PAMORA, patients must have been on opioids for a minimum of four weeks to be considered candidates.7 Before initiation of PAMORAs, all maintenance laxatives should be discontinued.3
Methylnaltrexone (Relistor®), as its name implies, is an N-methylated version of naltrexone that does not cross the blood-brain barrier. Patients may see a response to the medication within four hours of administration, while others may require repeat dosing if receiving higher daily morphine equivalencies.13 It is administered at 450 mg orally once daily or 12 mg subcutaneously once daily, with dose adjustments for moderate to severe renal impairment. Patients should be instructed to take oral methylnaltrexone with water at least 30 minutes before their first meal of the day. Subcutaneous methylnaltrexone injection sites should be rotated daily and administered in the upper arm, abdomen, or thigh.11
Subcutaneous methylnaltrexone is also indicated for OIC in patients receiving palliative care with inadequate laxative response and is dosed based on weight in this population.7,11 Patients may experience mild to moderate abdominal pain and flatulence.13 The use of methylnaltrexone for more than four months has not been studied, and use should therefore be limited to a short period of time.11
Naloxegol (Movantik®) is a pegylated derivative of naloxone which limits the drug’s ability to cross the blood-brain barrier.14 The effectiveness of naloxegol was determined in two phase 3 randomized control trials. For OIC, it is dosed 25 mg by mouth daily, and a dose reduction to 12.5 mg may be needed in those who do not tolerate the full dose.7 Dose reduction is required for renal impairment, as altered renal function can impair the clearance of naloxegol and lead to increased drug exposure and increased side effects. It should also be avoided in patients with severe hepatic impairment.
Patients should be instructed to take naloxegol on an empty stomach one hour before or two hours after their first meal of the day. This medication can be crushed and administered via a nasogastric tube. Naloxegol should not be used with strong cytochrome P450 3A4 (CYP3A4) inhibitors such as clarithromycin and ketoconazole, and dose adjustments should be made with moderate CYP3A4 inhibitors and CYP3A4 inducers.7 In addition, potential drug-drug interactions may occur with grapefruit due to hepatic drug metabolism via CYP3A4.7,15 Side effects appear to be dose related, with the most common being gastrointestinal: abdominal pain, diarrhea, nausea, and vomiting.14
Naldemedine (Symproic®) was approved by the Food and Drug Administration (FDA) in 2017 and is the newest PAMORA on the U.S. market. It acts on delta- and kappa-opioid receptors, in addition to mu-opioid receptors.9 Naldamedine is dosed at 0.2 mg by mouth once daily. Studies have demonstrated that impaired renal function does not require dose reductions, but naldemedine should not be used in patients with severe hepatic impairment as the drug’s safety has not been studied in this population.7,16 Patients can be instructed to take naldemedine with or without food.
Naldemedine is a major substrate of CYP3A4, P-glycoprotein, and uridine diphosphate-glucuronosyltransferase 1A3, which may lead to numerous potential drug-drug interactions.17 The use of strong CYP3A4 inducers (e.g., dexamethasone, carbamazepine, phenytoin, phenobarbital, rifampin) should be avoided, as they can reduce the effectiveness of naldemedine. Conversely, the concurrent use of moderate and strong CYP3A4 inhibitors can increase naldemedine levels, potentially leading to heightened adverse reactions. Drugs that inhibit P-glycoprotein can increase the bioavailability of naldemedine, whereas inducers may decrease it. Therefore, careful monitoring is recommended when these inducers and inhibitors are used alongside naldemedine.7
PAMORAs as a class are contraindicated in peptic ulcer disease, diverticulosis, colon cancer, and bowel obstruction.3 If a suboptimal response to PAMORAs occurs after three days, laxative therapies may be added to the patient’s regimen.3 The most common adverse effects associated with PAMORAs are gastrointestinal effects, including abdominal pain, flatulence, nausea, and diarrhea. If a patient experiences severe, persistent diarrhea or discontinues opioid therapy, PAMORAs should be halted.11
ADDITIONAL AGENTS
Lubiprostone (Amitiza®) is a type-2 chloride channel activator. It works by increasing the fluid secretion in the gastrointestinal tract and, as a result, increases tone, enhances peristalsis, and increases movement of the small bowel and colon.3 This medication was the first medication approved for OIC in adults taking opioids for non-cancer pain.18
Lubiprostone’s efficacy has not been established in patients taking methadone, and it is believed that the mechanism of action of methadone may cause lubiprostone to be ineffective.7 Recommended dosing for OIC is 24 mcg orally twice daily. Dose adjustments are required in moderate to severe hepatic impairment.19
Patients should be instructed to take lubiprostone with food and water to decrease nausea, a common side effect along with diarrhea and abdominal cramping.5,7,19 Patients who are also taking antihypertensive medications may experience dyspnea, syncope, and hypotension with the first dose, and taking this medication while seated may reduce the effects of orthostasis.
The risk of orthostasis may be increased in those experiencing diarrhea or vomiting. Trials of lubiprostone as long as 13 months have shown it to be well tolerated.7 The AGA Institute guidelines make no recommendations for the use of lubiprostone for OIC due to limited consistent evidence and low quality of evidence for use.12
Prucalopride (Motegrity®) is a selective serotonin type 4 receptor agonist that works by inducing giant migrating contractions and encouraging proximal colonic and gastropyloro-duodenal movement. In addition, patients with delayed gastric emptying may benefit from prucalopride, as it helps to increase gastric emptying.20 The FDA-approved indication for prucalopride is chronic idiopathic constipation, but this medication has been used off-label in OIC.20 It is dosed at 2 mg by mouth once daily for OIC, and dose adjustments should be made for severe renal dysfunction.
The use of prucalopride is contraindicated in intestinal perforation or obstruction, obstructive ileus, and severe inflammatory gastrointestinal tract conditions.4 The most common side effects are gastrointestinal upset, including abdominal pain, nausea, and diarrhea.4,20
Patients should be monitored for new or worsening suicidal ideations and depression, as well as severe, persistent diarrhea. If these develop, medication therapy should be stopped.4 Due to a lack of evidence to support its use, the AGA Institute guidelines make no recommendation for the use of prucalopride.12
CONCLUSION
OIC is a common side effect of opioids and should be prevented and treated promptly as it can lead to decreased quality of life. Initially, patients should use lifestyle modifications and laxatives to prevent OIC, although bulk-forming agents should be avoided due to the potential worsening of constipation. Patients who are refractory to traditional laxatives may trial PAMORAs after four weeks of opioid therapy.
These agents can cause undesired side effects of opioid withdrawal and gastrointestinal upset, and therefore patients should be monitored regularly while receiving these agents. Patients should stop treatment with PAMORAs if persistent or severe diarrhea occurs. Alternatively, lubiprostone or prucalopride can be used off-label to treat patients with OIC, although limited data exist for the use of these agents in this patient population.
Overall, a personalized approach that considers each patient’s unique circumstances, including their specific opioid regimen and overall health status, is essential for effectively managing OIC. Regular screening for OIC and a multifaceted approach involving dietary modifications, lifestyle changes, and pharmacological interventions can help optimize treatment outcomes and improve patients’ quality of life.
REFERENCES
1. Farmer AD, Holt CB, Downes TJ, Ruggei E, Del Vecchio S, De Giorgio R. Pathophysiology, diagnosis, and management of opioid-induced constipation. Lancet Gastroentrol Hepatol. 2018;3(3):203-212.
2. Saha S, Nathani P, Gupta A. Preventing opioid-induced constipation: a teachable moment. JAMA Intern Med. 2020;180(10):1371-1372.
3. Sizar O, Genova R, Gupta M. Opioid-induced constipation. In: Stat-Pearls. Treasure Island (FL): StatPearls Publishing; August 7, 2023.
4. Rome IV Criteria: C6, Opioid-Induced Constipation. The Rome Foundation. Accessed February 25, 2024. https://theromefoundation.org/rome-iv/rome-iv-criteria/
5. Andresen V, Layer P. Medical therapy of constipation: standards and beyond. Visc Med. 2018;34(2):123-127.
6. Rao VL, Micic D, Davis AM. Medical management of opioid-induced constipation. JAMA. 2019;322(2):2241-2242.
7. Gregorian T, Lewis J, Tsu L. Opioid-induced constipation: clinical guidance and approved therapies. US Pharm. 2017;42(12):15-19.
8. Gordon M. Nutrition tips for relieving constipation. Academy of Nutrition and Dietetics. Updated April 12, 2021. Accessed February 25, 2024. https://www.eatright.org/health/health-conditions/digestive-and-gastrointestinal/nutrition-tips-for-relieving-constipation
9. Kanemasa T, Koike K, Takase K, et al. Pharmacological profile of naldemedine, a peripherally acting mu-opioid receptor antagonist: comparison with naloxone and naloxegol. J Pharmacol Exp Ther. 2020;373(3):438-444.
10. Siegel JD, Di Palma JA. Medical treatment of constipation. Clin Colon Rectal Surg. 2005;18(2):76-80.
11. Methylnaltrexone. Lexi-Drugs. Hudson, OH: Lexicomp, 2024. Updated January 2024.
12. Crockett SD, Greer KB, Heidelbaugh JJ, Falck-Ytter Y, Hanson BJ, Sultan S. American Gastroenterological Association Institute guideline on the medication management of opioid induced constipation. Am J Gastroenterol. 2019;156(1):218-226.
13. Thomas J, Karver S, Cooney GA, et al. Methylnaltrexone for opioid-induced constipation in advanced illness. N Engl J Med. 2008;358(22): 2332-2343.
14. Chey WD, Webster L, Sostek M, et al. Naloxegol for opioid-induced constipation in patients with noncancer pain. N Engl J Med. 2014;370 (25):2387-2396.
15. Bui K, She F, Sostek M. The effects of renal impairment on pharmacokinetics, safety, and tolerability of naloxegol. J Clin Pharmacol. 2014;54(12):1375-1382.
16. Fukumura K, Yamada T, Yokota T, Kawasaki A. The influence of renal or hepatic impairment on pharmacokinetics, safety, and tolerability of naldemedine. Clin Pharmacol Drug Del. 2020;9(2):162-174.
17. Naldemedine. Lexi-Drugs. Hudson, OH: Lexicomp, 2024. Updated February 2024.
18. Wilson N, Schey R. Lubiprostone in constipation: clinical evidence and place in therapy. Ther Adv Chronic Dis. 2015;6(2):40-50.
19. Lubiprostone. Lexi-Drugs. Hudson, OH: Lexicomp, 2024. Updated January 2024.
20. Sloots CE, Rykx A, Cools M, Kerstens R, De Pauw M. Efficacy and safety of prucalopride in patients with chronic noncancer pain suffering from opioid-induced constipation. Dig Dis Sci. 2010;55(10):2912-2921.