There are some significant recent changes in the recommendations concerning intake of fats and how they can affect health. It’s a long story, so in this issue we’ll discuss saturated and unsaturated fats in all their combinations and varieties, and in the next issue we’ll recap the latest information about Omega 3 and Omega 6 fatty acids in the context of polyunsaturated fats.
Dietary fats are essentially triglycerides, that is fats based on a glycerol backbone (which consists of carbon, hydrogen, and oxygen) configured like the letter “E,” with three fatty acids comprising the three bars of the “E.” (Figure 1.) The fats found in all foods are a mixture of saturated (as in animal fat), monounsaturated (as in olive oil or peanut oil), and polyunsaturated (as in canola oil, corn oil, and soy bean oil), and food is classified according to the type of fat that predominates. Still, many of us may not remember all of the organic chemistry we learned many years ago, so in addition to reviewing current recommendations about fats in the diet, this article will also recap some basic chemistry about the chemical structure of fats. This is your chance to learn why fats are what they are – liquid, soft, or hard, and whether healthy or not.
Figure 1.
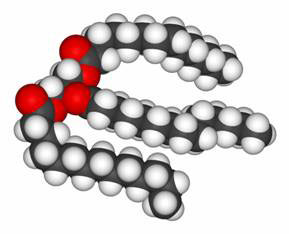 A Triglyceride Molecule
A Triglyceride Molecule
Trans Fats
Carbon atoms are tetravalent, forming covalent bonds with 4 other atoms, whereas hydrogen atoms form a bond with only one other atom. In saturated fatty acids, each carbon atom is connected to its two neighbor carbon atoms as well as to two hydrogen atoms, thus filling all four of its bonds (see Figure 2a.).
In unsaturated fatty acids some carbon atoms lack one hydrogen atom, and the unfilled bonds form a double bond with the adjoining carbon atom (see Figure 2b). In most naturally occurring unsaturated fatty acids, the single hydrogen atoms are on the same side of the carbon chain (cis configuration — meaning "on the same side" in Latin – see Figure 2b). Since the positive charges on the adjacent hydrogen atoms repel each other, the carbon chain is angulated like a hockey stick at the site of the double bond. These “hockey stick,” angulated carbon chains do not pack against each other well, which keeps the intermolecular forces weak, and makes unsaturated fats generally liquid at room temperature. Saturated fats (Figure 2a) have no angulated double bonds, and the molecules of straight carbon chains stack up neatly in a compact mass, making such fats solid.
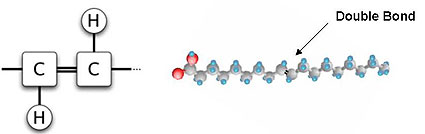
Hydrogenation solidifies fats by adding hydrogen atoms, which eliminate many of the angulating double bonds. Also, most of the new hydrogen atoms occupy the trans position, i.e. on “opposite” sides of the carbon atom (see Figure 2c), a configuration that eliminates angulations. Commercial hydrogenation is typically only partial, leaving some double bonds. This produces a malleable fat that is solid at room temperature, is excellent for baking, and melts with heating (or consumption). Not only does reducing the number of double bonds change the consistency of fatty acids, but it prolongs shelf life by making them less prone to rancidity. This favorable effect is due to the elimination of unsaturated double bonds, which is where the oxygenation occurs that causes fats to become rancid.
Figure 2A. - Saturated Fatty Acid
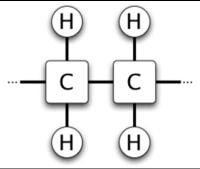
Figure 2B. - CIS - Unsaturated Fatty Acid
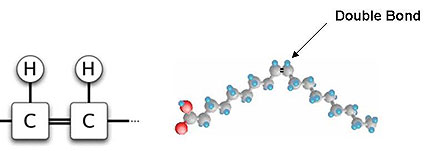
Figure 2C. - Trans - Unsaturated Fatty Acid
TRANS Fats and Health
When eaten, both saturated fats and trans fats raise LDL, but trans fats also lower HDL.
Even meat and dairy products from ruminant animals contain a small amount of trans fats that are manufactured by bacteria in the gut and absorbed. But after the process of partial hydrogenation of plant oils was commercialized in the form of Crisco in 1911, artificially produced fats became the major source of trans fats in the diet.
In the Nurses’ Health Study, those eating in the highest quartile of trans fats had blood levels of C-Reactive Protein 73% higher than those of the lowest quartile of trans fat consumption. The nurses’ risk of coronary heart disease roughly doubled for each 2% increase in trans fat calories consumed as a replacement for carbohydrate calories! By contrast, it takes a 15% increase in saturated fat calories (instead of carbohydrate calories) to produce a similar increase in risk of coronary disease. Replacing 2% of food energy from trans fat with non-trans unsaturated fats cuts the risk of coronary disease by 53%.1
There are also other drawbacks to trans fats. A recent study published this year in the Proceedings of the American Association of Cancer Research cites trans fats as increasing the risk of prostate cancer.2 Also, each 2% increase in intake of energy from trans unsaturated fats, as opposed to that from carbohydrates, was associated with a 73% risk of ovulatory infertility, according to an article in the American Journal of Clinical Nutrition earlier this year.3
Due to the health hazards of trans fats, researchers at the United States Department of Agriculture have investigated whether hydrogenation can be achieved without the side effect of trans fat production. They varied the pressure under which the chemical reaction was conducted — applying 200 psi of pressure to soybean oil while heating it to between 140 °C and 170 °C. This high-pressure modification reduced the trans fatty acid content of the resulting fat from about 40% by weight for the standard method to about 17% with high pressure. Blended with unhydrogenated liquid soybean oil, the high pressure processed oil produced margarine containing 5 to 6% trans fat, compared to about 15% in margarine with no modification of trans fat content. Based on current U.S. labeling requirements, the manufacturer could claim the product was free of trans fat. The level of trans fat may also be altered by modification of the temperature and the length of time during hydrogenation.
The bottom line with trans fats is to stay away from them as much as possible, including any food item that mentions the words “partial hydrogenated fats” or “partial hydrogenation”. Since the FDA allows foods with less than 0.5 grams per serving to be labeled as 0 grams per serving, the product label may say “0” trans fats, but the words “partial hydrogenation” are still in the list of ingredients. In that case, one should probably look for another food source.
Interesterified Fats
Interesterification is sometimes used as an alternative to partial hydrogenation. Although interesterified fats are not well known, these are oils like soybean oil that have been chemically modified to make them more stable. Interesterification substitutes a saturated fatty acid such as stearic acid on the middle limb of the “E” shaped glycerol backbone of the fat (see Figure 1). Unfortunately, these fats may pose health risks as bad or worse than trans fats. They have not yet been extensively studied, but they have already been cited to decrease HDLs more than trans fats. Also, some sources have stated that they can increase blood sugar. At present, it is best to avoid them.
Saturated Fats
Saturated fats consist of triglycerides that contain only saturated fatty acids, which means there are no double bonds between the carbons. Saturated fats are distinguished only by their differences in the number of carbon atoms (1-24). The higher saturated fats such as coconut oil and butter from cow’s milk are solid at room temperature, are more stable in cooking, and have longer shelf lives than unsaturated olive oil and other liquid vegetable oils. Their durability results from the absence of unsaturated bonds that can be oxidized, a process that turns the fat rancid. Foods like peanuts and natural peanut butter have no trans fats and high amounts of mono and polyunsaturated fats. A diet high in peanuts and natural peanut butter can decrease LDL and increase HDL. (Most popular commercial brands of peanut butter contain hydrogenated fat that is added to enhance smoothness and prevent separation of the liquid peanut oil.)
Some studies of the effects of saturated fat in the diet haven’t specifically analyzed contamination of saturated fats with trans fats. More research needs to be done, and some nutritionists feel that saturated fats may not be as bad as previously thought. Indeed, some saturated fats have nutritional value. Lauric acid, for example, has antimicrobial properties, which gives human milk its antimicrobial component, and may mean that lauric acid should be considered a conditional essential fatty acid.
Advanced Glycation Endproducts (AGEs)
Another confounding issue is the ingestion of exogenous advanced glycation endproducts (AGEs) which are common in browned processed foods such as bread crusts, or can result from cooking sugars and proteins together without substantial amounts of water. AGEs have the ability to form covalent crosslinks between proteins, which alters their structure and function in components of the cellular matrix, basement membranes, and vessel-walls. AGEs interact with a variety of AGE-binding receptors on cell surfaces, leading either to their endocytosis and degradation, or to cellular activation and pro-oxidant, pro-inflammatory events.
A large body of evidence suggests that AGEs are important pathogenetic mediators of almost all diabetes complications, i.e. both micro and macroangiopathies. For instance, AGEs are found in retinal vessels of diabetic patients, and their levels correlate with those in serum as well as with severity of the retinopathy.
A thorough review of this complex and important topic can be found in a publication of the American Diabetes Association.4
That’s enough about organic chemistry and formulas for one issue. Next time we’ll discuss polyunsaturated fats and Omega fatty acids.
REFERENCES
- http://www.answers.com/topic/trans-fatty-acid#wp-_note-12#wp-_note-12
- http://www.aacrmeetingabstracts.org/cgi/content/abstract/2006/1/943
- http://www.ajcn.org/cgi/content/abstract/85/1/231
- http://clinical.diabetesjournals.org/cgi/content/full/21/4/186
Alan S. Peterson, M.D.
Associate Director, Family & Community Medicine
Walter L. Aument Family Health Center
317 South Chestnut Street
Quarryville, PA 17566
717-786-7383
ASPeters@LGHealth.org