Summer 2024 - Vol. 19, No. 2
SCIENTIFIC REPORT
A Review of Novel Pharmacotherapeutic Agents for the
Management of Type 2 Diabetes Mellitus


Patterson Harrsch
Michelle Link Patterson, PharmD, BCACP
Felicia Harrsch, PharmD
Ambulatory Pharmacist Clinicians
Penn Medicine Lancaster General Health
The National Institute of Diabetes and Digestive and Kidney Diseases estimates the prevalence of diabetes in the United States was 37.3 million in 2019, and global estimates suggest 537 million adults live with diabetes.
1,2 Novel agents for managing diabetes are efficacious and offer cardiovascular (CV) and renal benefits that make them important in management. Additionally, ultra-long-acting and highly concentrated insulins make flexible dosing and lower injection volumes possible.
3,4
Studies demonstrate sodium-glucose cotransporter type 2 (SGLT2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists (RAs) can reduce major adverse cardiac events.
5-12 These are now considered first-line agents in patients with atherosclerotic cardiovascular disease or high CV risk, patients with established kidney disease, and those with heart failure, regardless of the glycosylated hemoglobin (HbA1C) at the initiation of therapy.
13
In addition, a novel GLP-1/glucose-dependent insulinotropic polypeptide (GIP) dual agonist, tripeptide, was approved by the Food and Drug Administration (FDA) in 2022. Despite final cardiovascular outcome trial (CVOT) data in progress, this agent has been shown to improve diabetes control and promote significant weight loss compared to other therapeutic options. A review of these agents follows.
SGLT2 INHIBITORS
SGLT2 inhibitors are relatively novel oral agents that have intermediate to high efficacy in lowering HbA1C. Currently available therapies include canagliflozin, dapagliflozin, and empagliflozin; new agents include bexagliflozin and ertugliflozin. SGLT2 inhibitors work in the apical membrane of the proximal tubule of the kidney, blocking glucose reabsorption from glomerular filtration by the SGLT2 receptor, reducing glycemia by causing glycosuria; this primarily affects fasting blood glucose.
14 Other than lowering HbA1C by approximately 1%, these once-daily medications modestly reduce body mass and blood pressure, thus addressing numerous comorbidities within the population affected by diabetes.
15
Due to efficacy and their ability to reduce CV and renal risks, SGLT2 inhibitors are now considered one of the first-line agents for type 2 diabetes mellitus (T2DM).
13 Cardiovascular benefits include decreased likelihood for heart failure-related hospitalizations, as well as a potential reduction in major cardiovascular events (MACE) and CV-related deaths. Renal benefits include decreased albuminuria, a reduced need for renal replacement therapy, stabilization of estimated glomerular filtration rate (eGFR), and reduced risk for disease progression.
5-8,12,15-19
Although there are eGFR cut-offs at which initiation of SGLT2 inhibitor therapy is not recommended, these agents may be continued until initiation of dialysis in those established on therapy.
20 Additionally, newer research proposes that, because SGLT2 inhibitors lower blood pressure without raising heart rate, they decrease sympathetic overactivity, subsequently causing reductions in blood pressure, heart rate, and edema, which may be the partial etiology of therapeutic benefit seen in heart failure.
16
SGLT2 inhibitors do have risks that may preclude their use. Due to the mechanism of action, SGLT2 inhibitors may cause acute kidney injury, volume depletion, and fluctuations in serum electrolytes, which is more important if patients are taking other antihypertensives or diuretics.
21-25 Electrolytes and renal function should be monitored at baseline and periodically during treatment.
These agents may cause an increased risk of diabetic ketoacidosis, and even euglycemic diabetic ketoacidosis, so it is recommended to hold therapy three to four days prior to planned surgeries depending on the agent.
26,27Additionally, they increase the risk of developing a urinary tract infection or genitourinary fungal infection; rarely, Fournier’s gangrene can occur.
21-25 Furthermore, canagliflozin, bexagliflozin, and ertugliflozin may increase the risk for lower limb amputation in clinical trials. Canagliflozin previously held a black box warning for lower limb amputations, but this was removed from the product labeling in 2020.
23
SGLT2 inhibitors can be used as monotherapy or add-on therapy. Cost may limit access.
 GLP-1 RECEPTOR AGONISTS
GLP-1 RECEPTOR AGONISTS
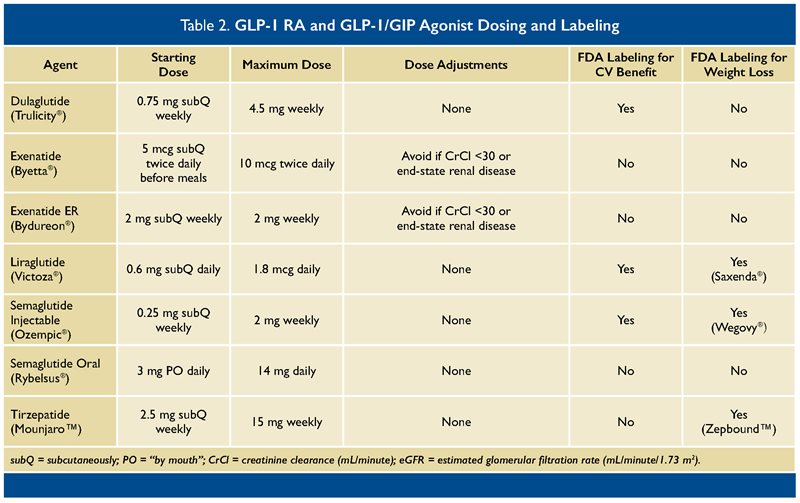
GLP-1 RAs are another burgeoning class of agents for the treatment of T2DM. Agents in this class include dulaglutide, exenatide, liraglutide, and semaglutide. While all these medications are given via subcutaneous injection, semaglutide is also available as an oral preparation. GLP-1 is an incretin hormone that increases glucose-dependent insulin secretion, decreases glucagon secretion, delays gastric emptying, and increases satiety, thereby decreasing food intake among other effects.
28
GLP-1 RAs enhance these pleiotropic effects and primarily act on post-prandial blood glucose. They are highly effective for the treatment of T2DM, with HbA1C reductions of 1% to 2%.
13,15 While native GLP-1 is typically short-lived due to enzymatic degradation by dipeptidylpeptidase-4 (DPP-4) and renal elimination, synthetic GLP-1 RA peptides have altered amino acid profiles that cause them to stay active longer, allowing for either daily or weekly administration.
28
GLP-1 RAs lower HbA1C and also have cardioprotective and renoprotective effects; therefore, they are also considered first-line options for T2DM depending on patient risks.
13 Specifically, these agents decrease the risk for MACE, including CV death, nonfatal myocardial infarction, and nonfatal stroke.
9-11,29 Renal benefits include reduced albuminuria, slowed decline in eGFR, as well as a reduced risk of renal replacement therapy.
30-33 With currently available data, the American Diabetes Association highlights dulaglutide, liraglutide, and semaglutide (injection) as having cardiac and renal benefits.
13 Though all of these agents share a similar mechanism of action, it is noteworthy that exenatide has a different chemical structure and is not approved for CV risk reduction.
In addition to cardiorenal benefits, numerous GLP-1 RAs also have been shown to produce at least a 5% weight reduction from baseline; liraglutide and semaglutide are each approved for weight loss at higher doses than used for T2DM.
34,35 When used with therapeutic lifestyle modifications, these medicines have been revolutionary in the management of patients who are overweight and obese. Studies demonstrate weight loss delays the progression from prediabetes to T2DM and improves glycemia, reducing the need for other glucose-lowering therapies.
13 In regard to further metabolic benefits, GLP-1 RAs may reduce morbidities in patients with non-alcoholic fatty liver disease.
13,36
The most recent GLP-1 RA to come to market is semaglutide, available as a once-weekly subcutaneous injection and a once-daily oral tablet.
37,38 With both formulations, the lowest dose is not considered an effective dose for blood glucose lowering and is meant as a “step up” to the next dose to limit potential side effects. It is unclear whether one formulation is more effective than the other, but both reduce HbA1C compared with placebo.
39,40
In numerous trials, weekly subcutaneous semaglutide 1 mg helped patients decrease their HbA1C by 1.5% to 1.8% compared with sitagliptin, liraglutide, exenatide extended release, dulaglutide, canagliflozin, or insulin glargine; various doses were used among the comparator medications.
41 In further trials, oral semaglutide 14 mg reduced HbA1C levels by 1% to 1.4% compared with sitagliptin or empagliflozin.
41 A randomized controlled trial demonstrated that subcutaneous semaglutide yields cardioprotection, and a pooled analysis of previous trials demonstrates it yields renoprotection as well.
10,33,42 Oral semaglutide is safe for use in moderate renal impairment and was non-inferior to placebo in terms of CV outcomes.
29,32 In obesity studies, both injectable and oral semaglutide significantly reduced body weight compared with placebo by at least 5% from baseline.
35,43
One of the primary challenges for patients with this class of medications is gastrointestinal side effects, such as nausea, vomiting, diarrhea, constipation, and slowed gastric emptying,
37,38,44-47 which can exacerbate gastroparesis and other gastrointestinal disorders. These side effects appear to be a class effect, likely partially due to the mechanism of action.
To combat this, it is recommended to start at the lowest dose and titrate slowly with at least four weeks between each dosing increase for once-weekly GLP1 medications. As noted above, the lowest doses of both oral and injectable semaglutide are meant as tolerability doses and are not expected to lower blood glucose, though they could have a mild effect in some patients.
Other potential adverse effects of this class include acute kidney injury in the setting of dehydration due to gastrointestinal side effects, gallbladder and biliary diseases, and acute pancreatitis, especially if the patient has comorbid hypertriglyceridemia. Additionally, GLP-1 RAs are contraindicated (black box warning) in patients with a personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2, as this is a rare, dose-dependent, and duration-dependent complication that appeared in mice and rats treated with GLP-1 RAs, with the exception of exenatide daily injection.
37,38,44-47
Lixisenatide was discontinued from the U.S. market in 2023 but is still available in a combination product with insulin glargine.
48 Fortunately, since these agents cause glucose-dependent insulin secretion, they have a low risk for causing hypoglycemia as monotherapy, which makes them a favorable option for many T2DM patients.
13
Generally, GLP-1 RAs are a highly effective option for T2DM, with numerous agents as options for co-morbid atherosclerotic CV disease or chronic kidney disease, as well as for providing dose-dependent weight reduction.
13 While cost may limit their use, GLP-1 RAs are another effective first-line option for T2DM, as either monotherapy or add-on therapy.
GLP/GIP DUAL RECEPTOR AGONIST
A newer, dual incretin (twincretin) protein agonist for GLP-1 and GIP, tirzepatide is now available for patients with T2DM. Similar to GLP-1 RAs, tirzepatide is a once-weekly subcutaneous injection, with its first dose as a “step-up” dose, not meant for providing glycemic control.
49
Tirzepatide works similarly to GLP-1 RAs, but with added GIP agonism, allowing for a synergistic effect on both glycemic control and weight reduction.
50,51 Theoretically, dual agonism of GLP-1 and GIP could make tirzepatide superior in HbA1C lowering compared with GLP-1 RAs, and trials suggest that it can lower HbA1C as much as 2%.
51
A head-to-head study compared three different doses of tirzepatide to 1 mg of semaglutide but did not include the maximum dose of semaglutide (2 mg); yet all three doses of tirzepatide used in the study were non-inferior and superior to 1 mg semaglutide for reductions in HbA1C.
52
Similar to its monotherapeutic target counterparts, tirzepatide can facilitate weight reduction of at least 5% from baseline and 15% on average.
50 In a head-to-head trial of tirzepatide versus semaglutide for diabetes, all three doses of tirzepatide resulted in more weight loss than 1 mg of semaglutide.
52
Tirzepatide was also approved in November 2023 for use in the treatment of patients diagnosed as overweight and obese. Unfortunately, there are no published data to elucidate CV and renal outcomes in patients taking tirzepatide. A study is ongoing, and this trial may help delineate whether tirzepatide has the same cardioprotective effects as other GLP-1 RAs.
INSULIN
While human insulin analogues have been available since 1982, there have been several updates in the last decade. Insulins are often categorized based on duration of action and concentration. In 2015, two new insulin preparations became available in the United States: insulin degludec (U-100 and U-200) and insulin glargine (U-300).
Insulin degludec is the first ultra-long-acting insulin available in the United States. It has a terminal half-life of approximately 25 hours and a duration of action exceeding 42 hours. Once injected, insulin degludec is slowly absorbed following zero-order kinetics, providing consistent glucose-lowering and low patient.to-patient pharmacokinetic variation. The extended duration of action allows for flexible dosing, meaning patients may wait 8 to 40 hours between doses without compromising patient safety.
3,53
A study of a forced-flexible dosing schedule for insulin degludec demonstrated similar safety and efficacy compared to standard dosing of insulin degludec and insulin glargine.
54 Participants in the forced-flexible dosing group administered insulin degludec on an alternating morning and evening schedule in which there was a minimum of eight hours and a maximum of 40 hours between injections. This highlights insulin degludec as a preferred basal insulin option for patients in which schedule conflicts or other barriers make it difficult to administer insulin at the same time each day.
Another benefit of insulin degludec is lower rates of nocturnal hypoglycemia.
55-58 A meta-analysis of seven clinical trials including over 3,300 participants with T2DM showed patients using this medication had lower rates of nocturnal hypoglycemia compared to patients using insulin glargine. In participants with T2DM not on bolus insulin, nocturnal hypoglycemia rates ranged from 6.1% to 20.4% with insulin degludec versus 8.8% to 24% with insulin glargine (rate ratio = 0.68; 95% CI = 0.57-0.82). Patients using insulin degludec also had a lower fasting plasma glucose.
58
Insulin glargine U-300 contains 300 units for every milliliter, compared to 100 units per milliliter for U-100 insulin glargine. This allows for decreased injection volumes and differences in pharmacokinetics and pharmacodynamics compared to U-100 insulin glargine. U-300 insulin glargine has an onset of action of six hours and duration of action of up to 36 hours, compared to U-100, which takes effect within three hours and lasts for up to 24 hours.
4
Despite having the same active ingredient as insulin glargine U-100, prescribers should be cautious when switching between products, as the dosing conversion is not necessarily 1:1. Thus when switching from insulin glargine U-100 to U-300, higher doses may be needed to achieve glycemic goals. In reverse, when switching from insulin glargine U-300 to U-100, the dose should initially be reduced by 20%. Dose titrations should be limited to every three to four days.
4
A study investigating the safety and efficacy of insulin glargine U-300 in patients with T2DM demonstrated patients needed higher doses of insulin glargine U-300 to achieve similar efficacy compared to U-100; however, hypoglycemia rates were similar or lower regardless of the definition of hypoglycemia. Despite higher insulin requirements in patients receiving U-300 compared to patients receiving U-100, participants receiving insulin glargine U-300 either lost more weight or gained less weight compared to those on U-100.
59-62
Several head-to-head trials have compared insulin glargine to insulin degludec. One such crossover study, in which patients used continuous glucose monitors, demonstrated that patients using insulin degludec U-100 had greater time in range and reduced incidences of hypoglycemia and nocturnal hypoglycemia.
63 A treat-to-target trial comparing insulin degludec U-200 to insulin glargine U-300 showed no difference in safety or rates of hypoglycemia.
64
Similarly, a 24-week trial comparing insulin glargine U-300 to insulin degludec U-100 showed similar improvements in HbA1C and rates of hypoglycemia. Mean insulin doses among patients using insulin glargine U-300 were higher than those in patients using insulin degludec U-100 (0.54 units/kg versus 0.43 units/kg, respectively).
65
Insulin degludec and insulin glargine are both valuable options for managing T2DM. More highly concentrated insulin degludec U-200 or insulin glargine U-300 are reasonable options if a patient’s insulin requirement exceeds 60 units per dose. Switching to these agents may improve adherence by reducing the need to split the basal insulin dose into two daily injections.
Patients in need of flexible insulin dosing may benefit from insulin degludec U-100 or U-200. Although clinical factors should be evaluated in determining the appropriate product, medication access and cost may influence insulin selection.
T2DM MEDICATION PIPELINE
Sotagliflozin is a novel dual SGLT1-SGLT2 inhibitor currently approved in the United States for use in heart failure, as well as for CV risk reduction in patients with T2DM and CKD (with or without albuminuria). It is available as 200 mg tablets, and the dose may be increased to 400 mg after at least two weeks.
66
In the European Union, sotagliflozin has already been approved in the treatment of type 1 diabetes mellitus.
67 The inhibition of SGLT1 in the intestines slows intestinal glucose absorption and is therefore a mechanism for reducing post-prandial glucose levels.
68,69
A study published in
Diabetes Care in 2022 comparing sotagliflozin to empagliflozin in patients with T2DM showed sotagliflozin reduced postprandial glucose, insulin, and GIP, and increased GLP-1. These benefits waned after lunch and dinner.
70 The mechanism for this change is not fully understood.
A 26-week phase III study of sotagliflozin 400 mg monotherapy in patients with T2DM not otherwise treated with antidiabetic therapy showed reductions in A1C of 1.03% compared to only 0.34% with placebo. Diarrhea, urinary tract infections, and headaches were the most reported adverse effects. At the time of this writing, this study was not yet published, but results of the trial are available on
clinicaltrials.gov (NCT02926937).
Danuglipron (PF-06882961) is a small-molecule, oral GLP-1 RA in development for the treatment of T2DM and obesity. At this time, only phase I and phase II studies have been published. A phase IIb study investigating danuglipron in patients with T2DM with or without metformin demonstrated it reduced A1C and fasting plasma glucose at 16 weeks at doses ranging from 2.5 mg to 120 mg. This medication is administered orally twice daily with food, and doses are escalated with a target dose of danuglipron 40 mg or more twice a day. Similar to other GLP-1 RAs, this medication can cause adverse effects such as nausea, diarrhea, and vomiting.
71
Insulin icodec is a novel basal insulin under investigation for use in both type 1 and type 2 diabetes. Its half-life exceeds 196 hours and reaches steady state after three to four weekly injections. Two trials of insulin icodec have been published. The first was a 26-week open label randomized controlled trial, which demonstrated that insulin-naïve participants had similar improvement in their HbA1C and no increased risks — that is, incidence of hypoglycemia — compared to decludec.
72,73
The second trial was a 26-week, randomized, open-label treat-to-target trial in which participants with baseline HbA1C from 7% to 10% were assigned to once-weekly icodec or once-daily insulin glargine U-100, which was combined with two to four daily bolus insulin injections. Insulin icodec was non-inferior to insulin glargine U-100 in HbA1C lowering at 26 weeks. Participants in the icodec group needed fewer bolus insulin doses and experienced similar rates of hypoglycemia compared to participants using insulin glargine U-100.
74
CONCLUSION
Type 2 diabetes mellitus remains a threat to global health despite the continued expansion of therapeutics available for its management. SGLT2 inhibitors, GLP-1 RAs, and a novel GLP/GIP agonist offer clinicians and patients many new options, while newer insulin formulations can change the landscape of T2DM management.
Additionally, several novel agents — including a dual SGLT1-SGLT2 inhibitor and a once-weekly basal insulin option — are in development.
KEY TAKEAWAYS
SGLT2 inhibitors have intermediate to high efficacy in lowering HbA1C.
GLP-1 RAs and SGLT2 inhibitors can be used as mono- or add-on therapy.
The altered amino acid profile of GLP-1 RAs allows them to stay active longer for weekly administration. They may produce at least 5% weight reduction from baseline.
Newer preparations of insulin may allow for weekly or even monthly administration.
REFERENCES
1. Diabetes Statistics. National Institute of Diabetes and Digestive and Kidney Diseases. September 15, 2019. Accessed July 28, 2023.
https://www.niddk.nih.gov/health-information/health-statistics/diabetes-statistics
2. Magliano DJ, Boyko EJ; IDF Diabetes Atlas 10th Edition Scientific Committee.
IDF Diabetes Atlas. 10th ed. International Diabetes Federation; 2021. Accessed April 11, 2024.
https://diabetesatlas.org/idfawp/resource-files/2021/07/IDF_Atlas_10th_Edition_2021.pdf
3. Tresiba
® (insulin degludec injection 100 U/mL, 200 UmL) [prescribing information]. Novo Nordisk; July 2022.
4. Toujeo
® (insulin glargine injection 300 units/mL) [prescribing information]. Sanofi-Aventis US; August 2022.
5. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes.
N Engl J Med. 2015;373(22):2117-2128.
6. Neal B, Perkovic V, Mahaffey K, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes.
N Engl J Med. 2017;377(7):644-657.
7. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy.
N Engl J Med. 2019;380(24):2295-2306.
8. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes.
N Engl J Med. 2019;380(4):347-357.
9. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes.
N Engl J Med. 2016;375(4):311-322.
10. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes.
N Engl J Med. 2016;375(19):1834-1844.
11. Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial.
Lancet. 2019;394(10193):121-130.
12. Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes.
N Engl J Med. 2020;383(15):1425-1435.
13. ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2023.
Diabetes Care. 2023;46(Suppl 1):S140-S157.
14. Ghezzi C, Loo DDF, Wright EM. Physiology of renal glucose handling via SGLT1, SGLT2 and GLUT2.
Diabetologia. 2018;61(10):2087-2097.
15. Tsapas A, Averinos I, Karagiannis T, et al. Comparative effectiveness of glucose-lowering drugs for type 2 diabetes.
Ann Intern Med. 2020;173(4):278-286.
16. Raza S, Osasan S, Sethia S, et al. A systematic review of sodium-glucose cotransporter 2 (SGLT2) inhibitors and sympathetic nervous system inhibition: an underrated mechanism of cardiorenal protection.
Cureus. 2022;14(6):e26313.
17. Grunberger G, Camp S, Johnson J, et al. Ertugliflozin in patients with stage 3 chronic kidney disease and type 2 diabetes mellitus: the VERTIS RENAL randomized study.
Diabetes Ther. 2018;9(1):49-66.
18. Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease.
N Engl J Med. 2020;383(15):1436-1446.
19. Wanner C, Inzucchi SE, Lechin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes.
N Eng J Med. 2016;375(18):232-334.
20. Navaneethan SD, Zoungas S, Caramori ML, et al. Diabetes management in chronic kidney disease: synopsis of the KDIGO 2022 clinical practice guideline update.
Ann Intern Med. 2023;176(3):381-387.
21. Brenzavvy
® [package insert]. TheracosBio; 2023.
22. Farxiga
® [package insert]. AstraZeneca Pharmaceuticals; 2020.
23. Invokana
® [package insert]. Janssen Pharmaceuticals; 2017.
24. Jardiance
® [package insert]. Boehringer Ingelheim Pharmaceuticals; 2022.
25. Steglatro
® [package insert]. Merck & Co. and Pfizer; 2021.
26. FDA Revises Labels of SGLT2 Inhibitors for Diabetes to Include Warnings About Too Much Acid in the Blood and Serious Urinary Tract Infections. Food and Drug Administration. Updated March 16, 2022. Accessed July 12, 2023.
https://www.fda.gov/drugs/drug-safety-and-availability/fda-revises-labels-sglt2-inhibitors-diabetes-include-warnings-about-too-much-acid-blood-and-serious
27. Ogawa W, Sakaguchi K. Euglycemic diabetic ketoacidosis induced by SGLT2 inhibitors: possible mechanism and contributing factors.
J Diabetes Investig. 2016;7(2):135-138.
28. Müller TD, Finan B, Bloom SR, et al. Glucagon-like peptide 1 (GLP-1).
Mol Metab. 2019;30:72-130.
29. Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes.
N Engl J Med. 2019;381(9):841-851.
30. Mann JFE, Ørsted DD, Brown-Frandsen K, et al. Liraglutide and renal outcomes in type 2 diabetes.
N Engl J Med. 2017;377:839-848.
31. Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial.
Lancet. 2019;394(10193):131-138.
32. Mosenzon O, Blicher TH, Rosenlund S, et al. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): a placebo-controlled, randomised, phase 3a trial.
Lancet Diabetes Endocrinol. 2019;7(7):515-527.
33. Shaman AH, Bain SC, Bakris GL, et al. Effect of the glucagon-like peptide-1 receptor agonists semaglutide and liraglutide on kidney outcomes in patients with type 2 diabetes: pooled analysis of SUSTAIN 6 and LEADER.
Circulation. 2022;145(8):575-585.
34. Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management.
N Engl J Med. 2015;373(1):11-22.
35. Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity.
N Engl J Med. 2021;384(11):989-1002.
36. Nevola R, Epifani R, Imbriani S, et al. GLP-1 receptor agonists in non-alcoholic fatty liver disease: current evidence and future perspectives.
Int J Mol Sci. 2023;24(2):1703.
37. Ozempic
® [package insert]. Novo Nordisk; 2017.
38. Rybelsus
® [package insert]. Novo Nordisk; 2021.
39. Sorli C, Harashima SI, Tsoukas GM, et al. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial.
Lancet Diabetes Endocrinol. 2017;5(4):251-260.
40. Aroda VR, Rosenstock J, Terauchi Y, et al. PIONEER 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes.
Diabetes Care. 2019;42(9):1724-1732.
41. Meier JJ. Efficacy of semaglutide in a subcutaneous and an oral formulation.
Front Endocrinol (Lausanne). 2021;12:645617.
42. Rossing P, Baeres FMM, Bakris G, et al. The rationale, design and baseline data of FLOW, a kidney outcomes trial with once-weekly semaglutide in people with type 2 diabetes and chronic kidney disease [published correction appears in Nephrol Dial Transplant. 2024 Mar 27;39(4):724].
Nephrol Dial Transplant. 2023;38(9):2041-2051.
43. Knop FK, Aroda VR, do Vale RD, et al. Oral semaglutide 50 mg taken once per day in adults with overweight or obesity (OASIS 1): a randomised, double-blind, placebo-controlled, phase 3 trial.
Lancet. 2023;402(10403):705-719.
44. Bydureon
® [package insert]. AstraZeneca Pharmaceuticals; 2018.
45. Byetta
® [package insert]. Amylin Pharmaceuticals; 2009.
46. Trulicity
® [package insert]. Eli Lilly and Company; 2017.
47. Victoza
® [package insert]. Novo Nordisk; 2017.
48. Adlyxin
® [package insert]. Sanofi-Aventis US; 2016.
49. Mounjaro
® [package insert]. Eli Lilly and Company; 2022.
50. Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity.
N Engl J Med. 2022;387(3):205-216.
51. Rosenstock J, Wysham C, Frías JP, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial.
Lancet. 2021;398(10295):143-155.
52. Frías JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes.
N Engl J Med. 2021;385(6):503-515.
53. Drab SR, Philis-Tsimikas A. A new option for glycemic control: insulin degludec, a new-generation basal insulin with an ultralong duration of action.
Pharmacotherapy. 2014;34(3):291-302.
54. Meneghini L, Atkin SL, Gough SC, et al. The efficacy and safety of insulin degludec given in variable once-daily dosing intervals compared with insulin glargine and insulin degludec dosed at the same time daily: a 26-week, randomized, open-label, parallel-group, treat-to-target trial in people with type 2 diabetes.
Diabetes Care. 2013;36(4):858-864.
55. Heller S, Buse J, Fisher M, et al. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN Basal-Bolus Type 1): a phase 3, randomised, open-label, treat-to-target non-inferiority trial.
Lancet. 2012;379(9825):1489-1497.
56. Garber AJ, King AB, Del Prato S, et al. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial.
Lancet. 2012;379(9825):1498-1507.
57. Zinman B, Philis-Tsimikas A, Cariou B, et al. Insulin degludec versus insulin glargine in insulin-naïve patients with type 2 diabetes: a 1-year, randomized, treat-to-target trial (BEGIN Once Long).
Diabetes Care. 2012;35(12):2464-2471.
58. Russell-Jones D, Gall MA, Niemeyer M, Diamant M, Del Prato S. Insulin degludec results in lower rates of nocturnal hypoglycaemia and fasting plasma glucose vs. insulin glargine: a meta-analysis of seven clinical trials.
Nutr Metab Cardiovasc Dis. 2015;25(10):898-905.
59. Yki-Järvinen H, Bergenstal RM, Bolli GB, et al. Less nocturnal hypoglycemia and weight gain with new insulin glargine 300 U/mL compared with 100 U/mL: 1-year results in people with T2DM using basal insulin with OADs (EDITION 2) [abstract].
Diabetes. 2014:63(suppl 1):LB23.
60. Bolli GB, Riddle MC, Bergenstal R, et al. New insulin glargine 300 U/mL. Glycemic control and hypoglycemia in insulin naïve people with type 2 diabetes (EDITION 3) [abstract].
Diabetes. 2014:63(suppl 1):A19.
61. Home PD, Bergenstal RM, Riddle MC, et al. Glycemic control and hypoglycemia with new insulin glargine 300 U/mL in people with T1DM (EDITION 4).
Diabetes. 2014:63(suppl 1):LB19-20.
62. Terauchi Y, Koyama M, Cheng X, Shimizu S, Hirose T; EDITION JP 2 Study Group. Glycemic control and hypoglycemia in Japanese people with T2DM receiving new insulin glargine 300 U/mL in combination with OADs (EDITION JP 2) [abstract].
Diabetes. 2014:63(suppl 1):LB24.
63. Goldenberg RM, Aroda VR, Billings LK, et al. Effect of insulin degludec versus insulin glargine U100 on time in range: SWITCH PRO, a crossover study of basal insulin-treated adults with type 2 diabetes and risk factors for hypoglycaemia.
Diabetes Obes Metab. 2021;23(11):2572-2581.
64. Rosenstock J, Cheng A, Ritzel R, et al. More similarities than differences testing insulin glargine 300 units/mL versus insulin degludec 100 units/mL in insulin-naive type 2 diabetes: the randomized head-to-head BRIGHT trial.
Diabetes Care. 2018;41(10):2147-2154.
65. Philis-Tsimikas A, Klonoff DC, Khunti K, et al; CONCLUDE Study Group. Risk of hypoglycaemia with insulin degludec versus insulin glargine U300 in insulin-treated patients with type 2 diabetes: the randomised, head-to-head CONCLUDE trial.
Diabetologia. 2020;63(4):698-710.
66. Inpefa
® (sotagliflozin) [prescribing information]. Lexicon Pharmaceuticals; May 2023.
67. Zynquista
® (sotagliflozin) [summary of product characteristics]. European Medicines Agency. 2019. Accessed August 27, 2023.
https://ec.europa.eu/health/documents/community-register/2019/20190426144497/anx_144497_en.pdf
68. Cariou B, Charbonnel B. Sotagliflozin as a potential treatment for type 2 diabetes mellitus.
Expert Opin Investig Drugs. 2015;24(12):1647-1656.
69. Powell DR, Zambrowicz B, Morrow L, et al. Sotagliflozin decreases postprandial glucose and insulin concentrations by delaying intestinal glucose absorption.
J Clin Endocrinol Metab. 2020;105(4):e1235-e1249.
70. Posch MG, Walther N, Ferrannini E, et al. Metabolic, intestinal, and cardiovascular effects of sotagliflozin compared with empagliflozin in patients with type 2 diabetes: a randomized, double-blind study.
Diabetes Care. 2022;45(9):2118-2126.
71. Saxena AR, Frias JP, Brown LS, et al. Efficacy and safety of oral small molecule glucagon-like peptide 1 receptor agonist danuglipron for glycemic control among patients with type 2 diabetes: a randomized clinical trial.
JAMA Netw Open. 2023;6(5):e2314493.
72. Philis-Tsimikas A, Bajaj HS, Begtrup K, et al. Rationale and design of the phase 3a development programme (ONWARDS 1-6 trials) investigating once-weekly insulin icodec in diabetes.
Diabetes Obes Metab. 2023;25(2):331-341.
73. Philis-Tsimikas A, Asong M, Franek E, et al. Switching to once-weekly insulin icodec versus once-daily insulin degludec in individuals with basal insulin-treated type 2 diabetes (ONWARDS 2): a phase 3a, randomised, open label, multicentre, treat-to-target trial [published correction appears in Lancet Diabetes Endocrinol. 2023 Jul;11(7):e9].
Lancet Diabetes Endocrinol. 2023;11(6):414-425.
74. Mathieu C, Ásbjörnsdóttir B, Bajaj HS, et al. Switching to once-weekly insulin icodec versus once-daily insulin glargine U100 in individuals with basal-bolus insulin-treated type 2 diabetes (ONWARDS 4): a phase 3a, randomised, open-label, multicentre, treat-to-target, non-inferiority trial.
Lancet. 2023;401(10392):1929-1940.