Spring 2024 - Vol. 19, No.1
SCIENTIFIC REPORT


Angstadt Scarborough
Rachel Angstadt, DO
Palliative Medicine Specialist
Penn Medicine Lancaster General Health
Bethann Scarborough, MD
Medical Director of Palliative Care
Penn Medicine Lancaster General Health
INTRODUCTION
Chronic pain is widespread, negatively impacts multiple aspects of life, and often coexists with depression and other chronic illnesses.
1 The complex, maladaptive pathophysiology of chronic pain complicates its management, and our society is still recovering from clinicians’ widespread misunderstanding regarding long-term opioid therapy and best practices to effectively manage chronic pain.
2
The prevalence of chronic pain is projected to continue to increase.
2,3 Older adults who live with chronic serious illnesses risk debilitation from comorbid pain, depression, and loneliness.
4 While pain in nonmalignant illnesses is often under-recognized, the prevalence of pain in these chronic conditions can match or exceed the prevalence of pain in people with cancer.
Primary care practitioners provide vital care for patients who can live for years with multiple chronic conditions. It is therefore necessary for all clinicians, particularly those in primary care settings, to comfortably manage patients with chronic pain. Clinicians can leverage low-risk, effective pharmacologic management options to improve the health of the most vulnerable members of our community. Part of the medical community’s service is to work to ensure individuals have a quality of life that is acceptable to them as they live with chronic comorbid conditions.
The following cases demonstrate that duloxetine can improve symptom control in ambulatory palliative care.
CASE 1
A 58-year-old male with newly diagnosed metastatic renal cell carcinoma to the bone presents to a palliative care clinic with uncontrolled left hip pain. He reports more than a year of distressing workup prior to the cancer diagnosis.
At the initial clinic visit, the patient is already taking acetaminophen 500 mg four times daily as needed, gabapentin 300 mg twice daily, and citalopram 20 mg daily as needed. He recently discontinued morphine, as its use led to worsening pain and headache.
The exam is significant for reproducible pain at the left hip that radiates to the left leg. He is tearful, due to both hip pain and the emotional weight of his diagnosis.
At the initial visit, he is advised to begin taking duloxetine 30 mg daily and oxycodone 10 mg every four hours as needed. This regimen is selected to target both the depressive symptoms and pain, and to ensure an as-needed medication is available for breakthrough pain.
At his one-week follow-up appointment, the patient reports improved pain and less tearfulness with adherence to duloxetine and use of oxycodone three times per day. The regimen has been well tolerated, yet he feels his symptoms could be better managed. Thus, the duloxetine is increased to 60 mg daily.
At a subsequent follow-up visit one month later, the patient reports improved mood; however, he is experiencing even more pain as his disease progresses. The duloxetine dose is now at the highest recommended dose for cancer-associated pain, therefore higher opioid doses will be used to further control his pain.
CASE 2
A 66-year-old woman with lung cancer presents to a palliative care clinic with persistent, generalized anxiety despite adherence to longstanding duloxetine 60 mg daily and use of diazepam 2.5 mg. The latter was prescribed twice daily as needed, and she reports using it several times per week. She denies pain or other comorbid symptoms.
A plan is initiated to transition her to a selective-serotonin reuptake inhibitor (SSRI) due to inadequate control of anxiety. She is thus started on sertraline 25 mg daily to trial tolerability; one week later, she reports no adverse effects. Her duloxetine is therefore reduced to 30 mg daily, and sertraline is increased to 50 mg to initiate a cross-taper; this plan is enacted to forgo precipitating serotonin-norepinephrine reuptake inhibitor (SNRI) discontinuation syndrome.
One month later, she returns to the clinic and reports her anxiety is improved; however, she is reporting paresthesia. She had forgotten the duloxetine was also intended to control neuropathy. To dually manage both symptoms with one agent, the SSRI is discontinued and her duloxetine is increased back to 60 mg.
Two weeks later, her neuropathy has improved but her anxiety is worse. Thus, duloxetine is increased to 90 mg daily.
She returns to the clinic two months later and reports she can now leave the house without anxiety attacks for the first time in years and has discontinued use of diazepam. Her husband reports her anxiety is the best it has been in over 20 years and their quality of life has significantly improved.
Over the next eight months she maintains excellent control of neuropathic pain, anxiety, and functional status without use of any opioid or benzodiazepine.
DISCUSSION
Health care practitioners often treat patients with comorbid chronic pain and psychiatric diagnoses. The use of non-sedating adjuvants in this population is clinically relevant as we continue to learn more about the risks of long-term opioid therapy, particularly when co-prescribed with other potentially sedating drugs.
Duloxetine treats a wide range of symptoms and can improve quality of life and functional status. It is an excellent tool to target multiple clinical syndromes, thereby reducing polypharmacy and subsequently decreasing side effect burden.
Duloxetine was first approved by the Food and Drug Administration in 2004 to treat major depressive disorder, and in the next decade it was subsequently approved to treat anxiety.
5 Depression and anxiety are associated with dysregulation of serotonergic and noradrenergic pathways; duloxetine is utilized in treatment of both disorders.
6 Patients treated with duloxetine have improved Hamilton Depression Rating Scale scores, as well as longer duration of recovery between exacerbations of depression, which allows for improved quality of life.
In addition, treatment with duloxetine leads to improved Hamilton Anxiety Rating Scale scores in patients with generalized anxiety disorder.
6 Further studies are needed to identify if this benefit persists in more modern anxiety measurement scales, such as the General Anxiety Disorder-7.
Duloxetine also improves symptoms in chronic pain disorders, and can effectively treat both painful diabetic neuropathy and fibromyalgia.
7 Patients with osteoarthritis of the knee report a significant improvement in Patient Acceptable Symptom State (PASS).
8 This is clinically significant because the PASS score evaluates overall patient well-being and acceptability of treatment. When used off-label to treat chemotherapy-induced peripheral neuropathy — a notoriously difficult-to-manage sequela — duloxetine can decrease pain while also improving numbness and tingling in the feet.
9,10
As its name implies, duloxetine reduces serotonin and norepinephrine reuptake. It also inhibits dopamine uptake in the prefrontal cortex, which has an impact on the descending spinal pathway of the dorsal horn, decreasing the perception of pain.
9 Doses generally begin with 30 mg daily and may be increased to 60 mg daily after one week. Doses up to 120 mg have been used in some pain syndromes with few adverse effects noted.
8
Although it has not been studied as extensively for treatment of cancer-related pain, anecdotal evidence suggests duloxetine may help patients with neuropathic cancer-related pain. Adding duloxetine to pregabalin and opioid regimens improves pain more than utilizing pregabalin and opioids alone.
11 This may allow a clinician to reduce opioids and the risk of pharmacologic side effects — and improve quality of life — in this population.
Duloxetine’s most promising benefit is its ability to improve overall functional ability and quality of life in all the above-mentioned syndromes. Patients with depression may have improved functional ability with duloxetine treatment.
12 Patients with generalized anxiety disorder may have improved functional status within many social settings (e.g., work and school, social life, leisure activities, and family and home responsibilities).
6
In addition to improving pain, duloxetine may also improve perceived daily functional status, quality of life, and use of ancillary analgesics.
10 Duloxetine seems to improve overall physical functioning regardless of the tool utilized to measure function, and may improve overall mental function and ratings of well-being.
13 These may be the most critically important aspects to an individual patient when they weigh whether to continue a prescription medication. Further studies should be completed to assess improvement in quality of life and functional status in patients with combined chronic pain and psychiatric illness.
Potential drawbacks to duloxetine include its side effect profile (i.e., nausea, diarrhea, and paradoxical agitation
6) and the need to taper prior to discontinuation. The risk of SNRI discontinuation syndrome is particularly important in patients who may lose the ability to tolerate capsules by mouth. Discontinuation due to side effects is unlikely; in general, the side effect burden is small enough to manage by splitting the dose into twice-daily dosing or controlling side effects with other as-needed medications.
7,11
Other adverse effects include hyponatremia (SIADH), hepatotoxicity, serotonin syndrome, suicidality (primarily in adolescents), and changes in libido.
10
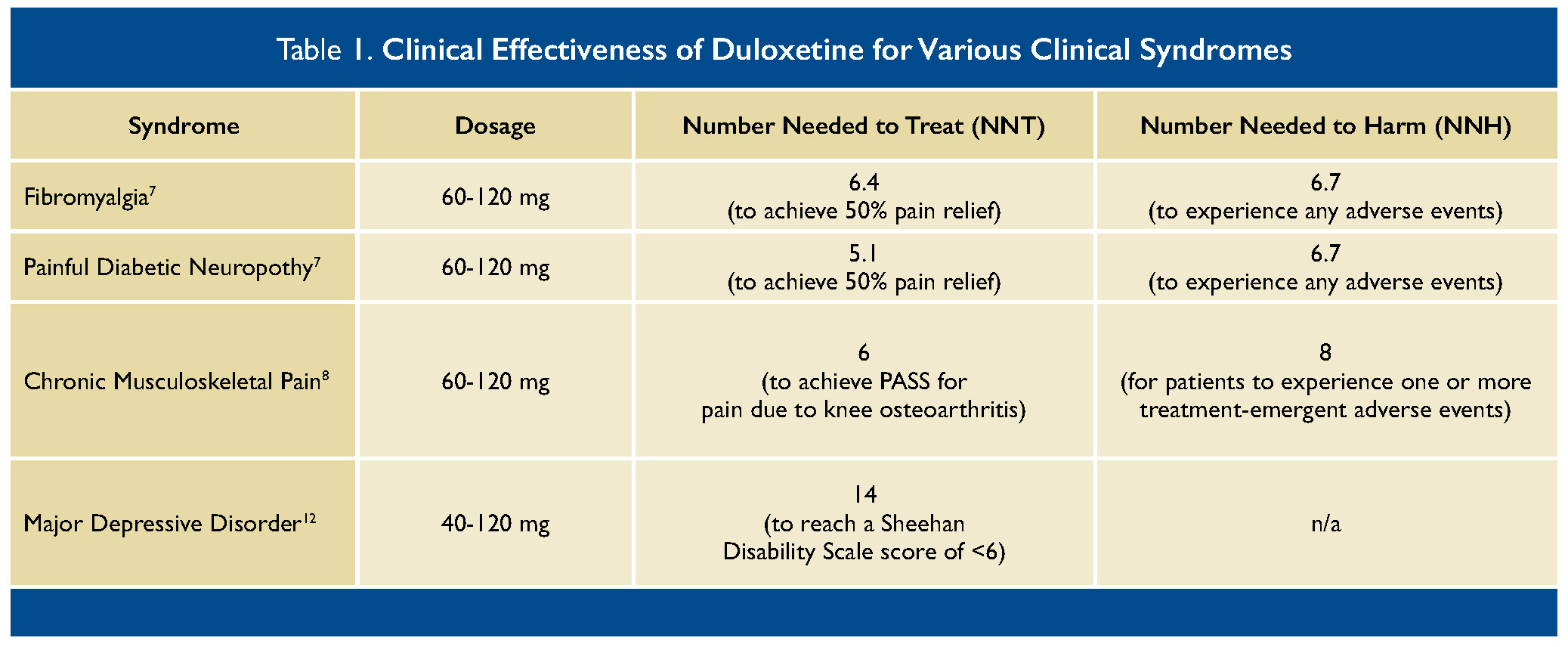
The Number Needed to Treat (NNT) versus Number Needed to Harm (NNH) are relatively similar (see Table 1). It is important to discuss risks and benefits with patients, who may agree that the benefits of improvement in pain, quality of life, and function outweigh the risk of gastrointestinal symptoms. Many patients find that adverse effects do not warrant stopping the medicine.
Smoking decreases the bioavailability of this medicine and may necessitate use of higher doses.
14 Co-administration of long-term medicines that inhibit cytochrome P450 CYP1A2 — of which there are many, including the SSRI fluvoxamine — increase the bioavailability and may necessitate lower doses of duloxetine.
14
It is worth bearing in mind that the overall improvement in pain scores with duloxetine are modest: this agent is probably safer than tricyclic antidepressants in elderly patients but otherwise should be thought of as comparable to amitriptyline for treatment of many chronic pain syndromes.
15 Data suggest that amitriptyline and duloxetine are essentially equivalent regarding their efficacy for diabetes-related neuropathy; low-dose dual therapy with both agents is an acceptable option as opposed to high-dose monotherapy for this syndrome.
16
CONCLUSION
An aging population is burdened with multi-morbid illnesses, however there are agents at hand to help manage chronic pain syndromes. Clinicians and patients must balance the risks of initiating long-term opioid therapy and take into account chronic pain, psychiatric comorbidities, and social determinants of health, all of which impact quality of life for members of our community. Clinicians must therefore apply an understanding of this complex biopsychosocial interplay to help their patients find safe, effective therapies to treat comorbid symptoms.
 REFERENCES
1.
REFERENCES
1. Rikard SM, Strahan AE, Schmit KM, Guy GP Jr. Chronic pain among adults — United States, 2019-2021.
MMWR Morb Mortal Wkly Rep. 2023;72(15):379-385.
2. Scarborough BM, Smith CB. Optimal pain management for patients with cancer in the modern era.
CA Cancer J Clin.2018;68(3):182-196.
3. Nahin RL, Feinberg T, Kapos FP, Terman GW. Estimated rates of incident and persistent chronic pain among US adults, 2019-2020.
JAMA Netw Open. 2023;6(5):e2313563.
4. Powell VD, Abedini NC, Galecki AT, Kabeto M, Kumar N, Silveira MJ. Unwelcome companions: loneliness associates with the cluster of pain, fatigue, and depression in older adults.
Gerontol Geriatr Med. 2021;7:2333721421997620.
5. [FDA approval for the antidepressive drug Cymbalta].
Krankenpfl J. 2004;42(5-6):154.
6. Rynn M, Russell J, Erickson J, et al. Efficacy and safety of duloxetine in the treatment of generalized anxiety disorder: a flexible-dose, progressive-titration, placebo-controlled trial.
Depress Anxiety. 2008;5(3):182-189.
7. Sultan A, Gaskell H, Derry S, Moore RA. Duloxetine for painful diabetic neuropathy and fibromyalgia pain: systematic review of randomised trials.
BMC Neurol. 2008;8:29.
8. Hochberg MC, Wohlreich M, Gaynor P, Hanna S, Risser R. Clinically relevant outcomes based on analysis of pooled data from 2 trials of duloxetine in patients with knee osteoarthritis.
J Rheumatol. 2012;39(2):352-358.
9. Dhaliwal JS, Spurling BC, Molla M. Duloxetine. In:
StatPearls. Treasure Island (FL): StatPearls Publishing; May 29, 2023.
10. Smith EM, Pang H, Cirrincione C, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial.
JAMA. 2013;309(13):1359-1367.
11. Matsuoka H, Iwase S, Miyaji T, et al. Additive duloxetine for cancer-related neuropathic pain nonresponsive or intolerant to opioid-pregabalin therapy: a randomized controlled trial (JORTC-PAL08).
J Pain Symptom Manage. 2019;58(4):645-653.
12. Sheehan DV, Mancini M, Wang J, et al. Assessment of functional outcomes by Sheehan Disability Scale in patients with major depressive disorder treated with duloxetine versus selective serotonin reuptake inhibitors.
Hum Psychopharmacol. 2016;31(1):53-63.
13. Skljarevski V, Zhang S, Iyengar S, et al. Efficacy of duloxetine in patients with chronic pain conditions.
Curr Drug Ther. 2011;6(4):296-303.
14. Knadler MP, Lobo E, Chappell J, Bergstrom R. Duloxetine: clinical pharmacokinetics and drug interactions. C
lin Pharmacokinet. 2011;50(5):281-294.
15. de Farias ÁD, Eberle L, Amador TA, da Silva Dal Pizzol T. Comparing the efficacy and safety of duloxetine and amitriptyline in the treatment of fibromyalgia: overview of systematic reviews.
Adv Rheumatol. 2020;60(1):35.
16. Tesfaye S, Sloan G, Petrie J, et al. Comparison of amitriptyline supplemented with pregabalin, pregabalin supplemented with amitriptyline, and duloxetine supplemented with pregabalin for the treatment of diabetic peripheral neuropathic pain (OPTION-DM): a multicentre, double-blind, randomised crossover trial [published correction appears in Lancet. 2022 Sep 10;400(10355):810].
Lancet. 2022;400(10353):680-690.