Summer 2022 - Vol. 17, No. 1
SCIENTIFIC REPORT
Mothers with Fetal Demise
Two Cases of SARS-CoV-2 Infection with Associated Placental Pathology
 Avery Briguglio
Penn Medicine Lancaster General Health Research Institute
Miguel Leandro Rufail, MD, PhD; Bruce E. King, MD;
Avery Briguglio
Penn Medicine Lancaster General Health Research Institute
Miguel Leandro Rufail, MD, PhD; Bruce E. King, MD;
Christina Colette Pierre, PhD
Penn Medicine Lancaster General Health Pathology
Michael D. Feldman, MD, PhD; Kathleen T. Montone, MD
Hospital of the University of Pennsylvania Pathology and Laboratory Medicine
The novel coronavirus SARS-CoV-2, responsible for the COVID-19 disease, has evolved into a worldwide pandemic. Although there has been much research on the origin, pathophysiology, treatment, and immunization of SARS-CoV-2, only a limited number of studies consider its effects on maternal health and fetal development, particularly in the second trimester of pregnancy.1-5
We present two similar cases of intrauterine fetal demise (IUFD) occurring during the second trimester of pregnancy in the setting of a SARS-CoV-2 positive mother.
CLINICAL PRESENTATION
Case 1
A 21-year-old unvaccinated gravida 2 para 1 woman (BMI 29.51 kg/m2) presented to Penn Medicine Lancaster General Health Women & Babies Hospital triage at 21 weeks gestational age. Her past medical history included anemia, headaches, a prior detached retina, and frequent tobacco use. (She reported quitting during the fourth week of her pregnancy.) She complained of leakage of fluid and contractions, but otherwise had no symptoms of SARS-CoV-2 infection. As part of the hospital protocol during the COVID-19 pandemic, the patient underwent a nasopharyngeal PCR screen and was found to be positive.
Although ultrasound one week prior had been normal, an ultrasound at this presentation confirmed fetal demise. She underwent induction with misoprostol and oxytocin, and she delivered a male fetus and placenta vaginally after six hours of labor without complications. Her vitals were normal following delivery. She remained asymptomatic from her SARS-CoV-2 infection during her delivery and hospitalization. At the patient’s request, a fetal autopsy and placental evaluation were conducted.
Case 2
A 29-year-old G1P0 female presented to Women & Babies Hospital triage at 22 weeks gestational age with complaint of a gush of blood-tinged fluid. She was unvaccinated for SARS-CoV-2, and other noteworthy history showed that starting two weeks prior to admission she had symptoms of congestion, fatigue, dry cough, diaphoresis, chills, and increased dyspnea, especially with exertion. Ten days prior to presentation to triage, the patient tested positive for SARS-CoV-2 by nasopharyngeal PCR swab. No respiratory assistance was needed to treat the dyspnea on exertion. She tested negative for SARS-CoV-2 by nasopharyngeal PCR one week later (three days prior to presentation to triage).
On presentation to triage, the patient was found to have preterm premature rupture of the membranes (PPROM); IUFD was confirmed by ultrasound. The patient underwent induction with misoprostol and delivered a male fetus and placenta vaginally after six hours of labor without complications. At the patient’s request, a fetal autopsy was not conducted, however a gross external fetal evaluation was performed during the placental examination.
PLACENTAL EXAMINATION
Both placentas were submitted to the pathology laboratory in formalin and processed according to standard procedures; gross and histological examination were performed. In each case, the placental disc was at the high end of expected range (>95th percentile): the first case weighed 153 grams (96th percentile for gestational age) and the second case 175 grams (97th percentile for gestational age).
Six representative unstained placental sections of the first case were sent to the pathology laboratory at the Hospital of the University of Pennsylvania to undergo chromogenic in situ hybridization (ISH) and complement 4d (C4d) immunostaining. ISH staining confirmed the presence of SARS-CoV-2 within the syncytiotrophoblast (Fig. 1a).
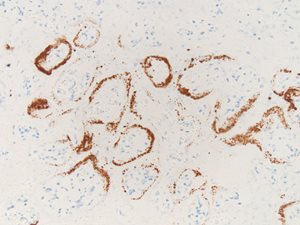
Fig. 1a. ISH for SARS-CoV-2 virus showing viral RNA within syncytiotrophoblast (Case 1).
Complement staining of C4d was found in the syncytiotrophoblastic layer of the placental villi (Fig. 1b).
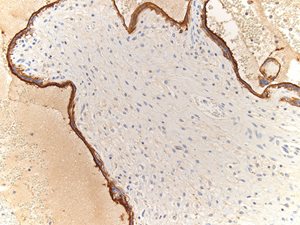
Fig. 1b. Immunostaining of C4d in syncytiotrophoblastic layer of placental villi (Case 1).
This C4d staining of the placenta was much stronger and more uniform in our case than in a second trimester IUFD control placenta (Fig. 1c).
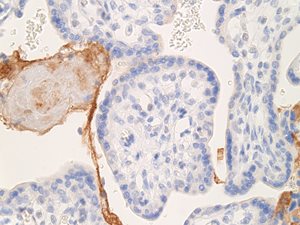
Fig. 1c. Immunostaining of C4d in second trimester IUFD placenta (control).
In both cases, placental histology showed extensive diffuse intervillous and perivillous fibrin deposition with widespread associated villous infarction and hemorrhage (Figs. 2a and 2b).
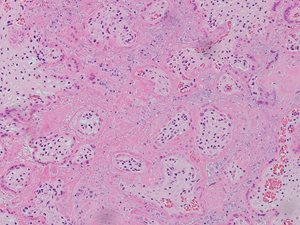
Fig. 2a. Extensive intervillous and perivillous fibrin deposition with associated villous infarction, 10X (Case 1).
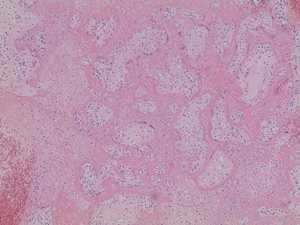
Fig. 2b. Extensive intervillous and perivillous fibrin deposition with associated villous infarction, 20X (Case 1).
In addition, significant thrombi deep to the fetal surface and patchy hemorrhage throughout the placental parenchyma were identified in both patients (Figs. 2c and 2d). No acute or chronic inflammation was noted in either case.

Fig. 2c. Gross examination of the placenta reveals patchy hemorrhage throughout the placental parenchyma (Case 1).
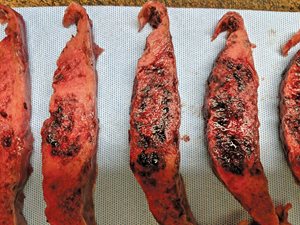
Fig. 2d. Gross examination of the placenta reveals patchy hemorrhage throughout the placental parenchyma (Case 2).
FETAL EXAMINATION
A fetal autopsy of the first patient showed no developmental abnormalities; however, tissue examination revealed degenerative changes consistent with IUFD without evidence of vasculopathy. The lung sections showed scattered degenerated cells (possibly neutrophils) within alveolar spaces. The umbilical cord (aside from being slightly edematous) and fetal membranes exhibited no specific histopathologic abnormalities.
The fetuses’ weights were low: in the first case, 222 grams (1.45 fetal-placental weight ratio, <5th percentile), and in the second case, 392 grams (2.24 fetal-placental weight ratio, <50th percentile). The fetal external measurements were within normal limits. The organ weights for the first case were slightly small for gestational age. In the second case, the fetus also showed no gross external abnormalities other than blood pooling in areas of the subcutaneous tissue and skin sloughing, indicating death several days prior.
No testing for SARS-CoV-2 was performed on either of the fetuses or amniotic fluids.
LABORATORY METHODS
Given the severity of the placental abnormalities observed in the first case, six representative unstained placental sections were sent to the pathology laboratory at the Hospital of the University of Pennsylvania for further analysis.
Chromogenic RNA ISH (BOND RNAscope Detection Reagents Brown; Leica Biosystems DS9790) was performed using a Leica Bond III instrument. Five-micron-thick FFPE tissue sections were stained according to manufacturer instructions with probes to SARS-CoV-2 viral RNA (Probe-V-nCoV2019-S; Advanced Cell Diagnostics 848568). Positive (PPIB; Leica Biosystems RS7755) and negative (dapB; Leica Biosystems RS77756) control probes were used to assess RNA and tissue quality.
The tissue sections were stained using polyclonal antibodies against anti-human C4d (diluted 1:100; American Research Products Inc., 12-5000). Staining performed with a Leica Bond III instrument used the Bond Polymer Refine Detection System (Leica Biosystems DS9800). Heat-induced epitope retrieval was done for 20 minutes in Epitope Retrieval Solution 1 (Leica Biosystems AR9961).
DISCUSSION
Although no formal diagnosis of maternal vascular malperfusion (MVM) occurred, these cases had many features consistent with MVM, such as gross findings of placental infarction and hemorrhage, and microscopic findings of extensive intervillous and perivillous fibrin deposition.3,6 These findings align with those described in much of the literature on placental pathology associated with SARS-CoV-2 infections.1-4 Further, although fetal vascular malperfusion (FVM) has been associated with COVID-19,1,2,7 there was no evidence of FVM in this case.
In addition, there was no evidence of any vasculopathy, which has been reported to be one of the main features of MVM associated with COVID-19.1-3 No acute or chronic inflammation was noted, which is consistent with a subset of SARS-CoV-2 positive pregnancies that exhibit no inflammatory response.1,2,8 Other studies have demonstrated clear evidence of inflammation in SARS-CoV-2 infection-associated pregnancies through pathologies such as villitis, intervillositis, deciduitis, chorioamnionitis, and funisitis.3,4,9 It is important to note that the lung sections collected during the fetal autopsy showed scattered collections of degenerated cells within the alveolar spaces, possibly representing neutrophils (and thus serving as potential evidence for inflammation).
Pregnant women have increased levels of ACE 2 receptors in the placenta to help facilitate fetal growth and regulate angiotensin-II levels. Due to the transmembrane ACE 2 providing a point of entry into cells, viruses such as SARS-CoV-2 can enter the syncytiotrophoblast, cytotrophoblast, endothelium, and vascular smooth muscle of villi where ACE 2 is significantly expressed.10 Given this virus’s association with the activation of coagulation pathways and potential progression to fibrinolysis,11 there is enough evidence to suggest hypercoagulopathy leading to thrombosis or increased fibrin deposition resulting in associated villous infarction or hemorrhage.1-4 These histopathological features appear to exist regardless of COVID-19 symptomatology.
Viral particles, such as SARS-CoV-2, can induce activation of the alternative and lectin complement pathways, causing the deposition of several complement proteins.12,13 One of these complement proteins, C4d, has been used as a biomarker to detect antibody-mediated disease, and it has applications for detecting antibody-mediated injury in placental tissue.14,15 Since covalently bound C4d has a greater chance of staying at the original site of complement activation than the antibodies themselves, and because it anchors tightly to the tissue, it was utilized for the immunostain over other complement proteins and immunoglobulins.14 Consequently, complement staining of C4d was performed to assess for immune-complex-mediated deposition.
In case 1, the syncytiotrophoblastic layer of the placental villi had evidence of significant and uniform C4d deposition, especially when compared to the control placenta. A related study also found C4d in the syncytiotrophoblastic layer of chorionic villi, with C4d expression being more significant in preeclampsia placenta relative to placenta lacking complications.15 It is important to note that while C4d deposition in the preeclampsia placenta was correlated with increased syncytial knots, it was not associated with any other histologic features.15
In addition, positive C4d staining (defined as >10% syncytiotrophoblast C4d immunoreactivity) has been associated with a lower gestational age at delivery, lower infant birth weight, and lower placental weight compared with cases showing no C4d staining.15 These factors could help explain the significant C4d deposition we saw in our case. Our results were unlike those of researchers who found similar complement staining patterns in placentas from patients who were either positive or negative for SARS.CoV-2 infection.12
When SARS-CoV-2 infects cytotrophoblasts and syncytiotrophoblasts, it elicits complement activation and triggers inflammatory cytokines, which are thought to cause a pro-coagulant environment. More specifically, the complement fixation along the villi border might damage the microvillous syncytiotrophoblastic membrane, which is then responsible for the complement activation and subsequent C4d deposition.16
Other researchers postulate that the C4d deposition along the microvillous border of the syncytiotrophoblasts may be responsible for the subsequent fibrin deposition and histiocytic influx.16 This may contribute to the histological features we saw in both cases, although only the first case underwent C4d immunostaining. Unfortunately, available literature regarding the use of C4d staining in SARS-CoV-2 affected placentas remains scarce.
CONCLUSION
ISH stain for C4d is more conclusive and widely used than C4d immunostain.17 In our case, it confirmed the presence of SARS-CoV-2 viral antigens in the placenta and adds evidence to the possibility of vertical transmission of SARS-CoV-2. C4d present in the placenta increases the likelihood that SARS-CoV-2 could have been involved in the histopathological findings presented and the eventual fetal demise in our case.
This study is subject to some limitations. First, we were unable to perform PCR testing on either of the fetuses and amniotic fluid to confirm vertical transmission of infection.8 In addition, the first patient’s past medical history of anemia and smoking can potentially serve as a contributing factor to the fetal outcome.
The extent of placental changes seen is the most logical contributing cause of fetal demise observed in both cases. Nonspecific, extensive intervillous fibrin deposition, as well as other features of MVM, have been seen in cases of active COVID-19,1-4 even in the setting of an asymptomatic mother.1,3
Consistent with our findings, it appears that pregnant women with SARS-CoV-2 infections, regardless of symptomatology, are more likely to exhibit adverse fetal outcomes and have more frequent placental histopathologic abnormalities.18,19 Researchers have found that compared to controls, women who had been infected were more likely to evolve to preterm labor and infant death, and their placentas were more likely to show features of MVM and FVM.18
Recent publications demonstrate a greater rate of placental histopathologic abnormalities among patients with a history of SARS-CoV-2 infection compared to controls, especially FVM and villitis of unknown etiology, although they did not observe an increase in other placental histopathologic lesions.19 Other researchers have identified similar fetal and maternal outcomes between infected and uninfected groups, as well as no significant difference in observed placental gross or microscopic morphology.20
This case discussion highlights the need for more studies to further establish the clinicopathological correlations of SARS-CoV-2 infection during pregnancy. Doing so would enable providers to better understand their implications as they pertain to maternal health and fetal growth, and to help facilitate improved perinatal outcomes. Until then, increased antenatal surveillance and specialized care is needed for pregnant women diagnosed with SARS-CoV-2.
ACKNOWLEDGEMENTS
Avery Briguglio’s co-authors would like to acknowledge his efforts in the writing of this article. Avery is a Temple University graduate who was recently accepted to the medical school at the Penn State College of Medicine in Hershey. The authors also thank Amy Ziober for providing the standard operating procedures for the immunostains performed at the Hospital of the University of Pennsylvania.
REFERENCES
1. Shanes ED, Mithal LB, Otero S, Azad HA, Miller ES, Goldstein JA. Placental pathology in COVID-19. Am J Clin Pathol. 2020;154(1):23-32.
2. Sharps MC, Hayes DJL, Lee S, et al. A structured review of placental morphology and histopathological lesions associated with SARS-CoV-2 infection. Placenta. 2020;101:13-29.
3. Menter T, Mertz KD, Jiang S, et al. Placental pathology findings during and after SARS-CoV-2 infection: features of villitis and malperfusion. Pathobiology. 2021;88:69-77.
4. Richtmann R, Torloni MR, Oyamada Otani AR, et al. Fetal deaths in pregnancies with SARS-CoV-2 infection in Brazil: a case series. Case Rep Womens Health. 2020;27.
5.Baud D, Greub G, Favre G, et al. Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. JAMA. 2020;323(21):2198-2200.
6. Khong TY, Mooney EE, Ariel I, et al. Sampling and definitions of placental lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch Pathol Lab Med. 2016;140(7):698-713.
7. Wastnedge EAN, Reynolds RM, van Boeckel SR, et al. Pregnancy and COVID-19. Physiol Rev. 2020;101:303-318.
8. Hecht JL, Quade B, Deshpande V, et al. SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: a series of 19 placentas from COVID-19-positive mothers. Mod Pathol. 2020;33:2092-2103.
9. Hosier H, Farhadian SF, Morotti RA, et al. SARS-CoV-2 infection of the placenta. J Clin Invest. 2020;130(9):4947-4953.
10. Dhaundiyal A, Kumari P, Jawalekar SS, Chauhan G, Kalra S, Navik U. Is highly expressed ACE 2 in pregnant women “a curse” in times of COVID.19 pandemic? Life Sci. 2021;264:118676.
11. Ji HL, Zhao R, Matalon S, Matthay MA. Elevated plasmin(ogen) as a common risk factor for COVID-19 susceptibility. Physiol Rev. 2020;100(3): 1065-1075.
12. Mulvey JJ, Magro CM, Ma LX, Nuovo GJ, Baergen RN. Analysis of complement deposition and viral RNA in placentas of COVID-19 patients. Ann Diagn Pathol. 2020;46:151530.
13. Mondal R, Lahiri D, Deb S, et al. COVID-19: are we dealing with a multi-system vasculopathy in disguise of a viral infection? J Thromb Thrombolysis. 2020;50:567-579.
14. Cohen D, Colvin RB, Daha MR, et al. Pros and cons for C4d as a bio-marker. Kidney Int. 2012;81(7):628-639.
15. Choi SY, Kim KH, Lee M, Yeo MK, Kim J, Suh KS. Complement component C4d deposition in the placenta of preeclampsia patients and renal glomeruli in 1 postpartum renal biopsy. Appl Immunohistochem Mol Morphol. 2020;28(2):139-145.
16. Watkins JC, Torous VF, Roberts DJ. Defining severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) placentitis. Arch Pathol Lab Med. 2021;145(11):1341-1349.
17. Wong YP, Khong TY, Tan GC. The effects of COVID-19 on placenta and pregnancy: what do we know so far? Diagnostics (Basel). 2021;11(1):94.
18. Rebutini PZ, Zanchettin AC, Stonoga ETS, et al. Association between COVID-19 pregnant women symptoms severity and placental morphologic features. Front Immunol. 2021;12:685919.
19. Patberg ET, Adams T, Rekawek P, et al. Coronavirus disease 2019 infection and placental histopathology in women delivering at term. Am J Obstet Gynecol. 2021;224:382.e1-18.
20. Tasca C, Rossi RS, Corti S, et. al. Placental pathology in COVID-19 affected pregnant women: a prospective case-control study. Placenta. 2021;110:9-15.