Winter 2020 - Vol. 15, No. 4

 Eastman Kontra
Eastman Kontra
The Making of a Pandemic
Bubonic Plague in the 14th Century
James T. Eastman, MD. FCAP
Department of Pathology and Laboratory Medicine (retired)
Lancaster General Hospital
Edited and updated in 2020 by:
Joseph M. Kontra, MD
Division Chief, Infectious Diseases
Penn Medicine Lancaster General Health
Editor’s Note: This article originally appeared in the Spring 2009 issue of JLGH (Vol.4, No.1). It seems particularly timely to publish an updated version now, while the world copes with a different pandemic.
ABSTRACT
Bubonic plague—a fast-spreading, highly lethal infection caused by Yersinia pestis—is most commonly associated with the 14th century, when it wiped out at least one third of the entire Eurasian population with a rapidly progressive and often fatal illness characterized by lymphadenopathy, septicemia, and pneumopathic effects. The incidence and virulence of this disease has diminished over the years, being limited to well-circumscribed areas by the latter half of the 20th century. However, its recent re-emergence around the world, coupled with an expanded appreciation of its great genotypic and phenotypic diversity, has introduced the threat of multidrug resistance and a modern-day pandemic. This may be avoided through our continued efforts to develop more rapid diagnostic tests and strain-specific vaccines, as well as through improved physician awareness, patient education, and close monitoring of environmental and sociopolitical changes that could reintroduce the conditions that contributed to the “Black Death.”
INTRODUCTION
In October 1347, several Genoese ships pulled into port at Messina, Sicily. The harbormaster noticed crew members who were clearly ill disembarking, and quickly sent the ships away. It was too late; within a matter of days, people were dead or dying in the city.1,2 The plague had arrived in Europe.
Imagine the questions running through people's minds: What is this disease? Boils, gangrene, vomiting blood, madness—it must have seemed more like divine retribution than a potentially curable disease. Where did it come from? Perhaps from Caffa, a Genoese city in the Crimea that was attacked by Mongols who catapulted diseased corpses over the walls to kill or drive off the citizens within; perhaps from China, where a similar disease was seen years earlier;3, or from a port along a trade route between China and the Mediterranean.
Why does it kill so fast? Carts filled with dead bodies were wheeled past dead rats lining the city streets. But with no knowledge of bacteriology, the most learned medieval minds were unable to make a connection between the animal and the disease. Why were some sailors still alive? Many of the dead must have prayed to be similarly exempted.
This article provides modern answers to these questions, and offers lessons from this 14th century pandemic that can be used to avoid such devastation today.
THE PLAGUE: A BRIEF HISTORY
1347 C.E. was not the first time Europe had seen the plague, and it would not be the last. This was the second of three plague pandemics. The first originated in the Middle East circa 532 C.E., killing at least half of the population in its path, and fostering the widespread scapegoating and fear which characterized the Dark Ages that followed.
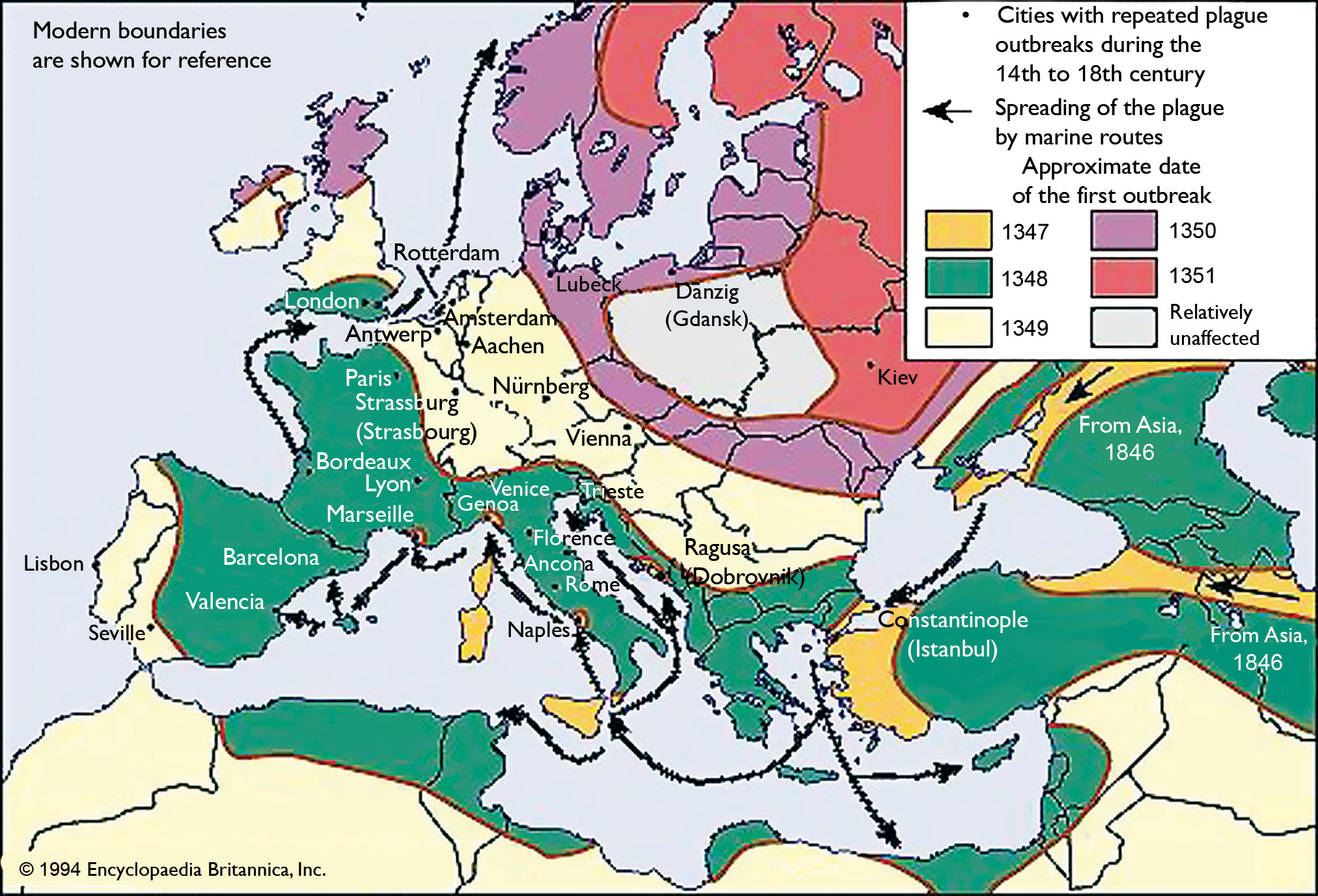
Fig.1. The path of plague through Europe during the 14th century. © 1994 Encyclopedia
Britannica Inc. Used with permission.
The second (the “Great Pestilence,” or “Black Death”) first appeared in China by 1334 C.E. and spread along trade routes to Europe, killing 20-30 million people (one third of the European population) between 1347 and 1351 (Fig. 1). The third pandemic began with small outbreaks in China around 1855 C.E. and exploded in 1894, wiping out as many as 12 million people. It then spread to 77 ports globally over the next 7 years. In all, an estimated 200 million people died during these three pandemics.3
Each pandemic was “extended" by periodic smaller outbreaks. The 14th century Black Death pandemic was followed by frequent epidemics through 1665, then less frequently into the 18th century.3 Unlike the main pandemic, the later outbreaks were limited to smaller foci and had progressively less virulent effects.
Currently, we are in an extended period of the third pandemic, characterized by an exponentially lower incidence of the disease (1,000-5,000 cases and 100-200 deaths annually over the past 20 years), confined mainly to Vietnam, India, and Africa.
In the United States, an average of 7 cases has been reported annually over the last few decades (range 1-17 cases/year), mostly in the Southwest, Utah, and California (Fig. 2), and usually during the warmer months.4 More recently, the appearance of cases on virtually every continent (Fig. 3) has triggered concerted efforts to improve treatment and prevention.5
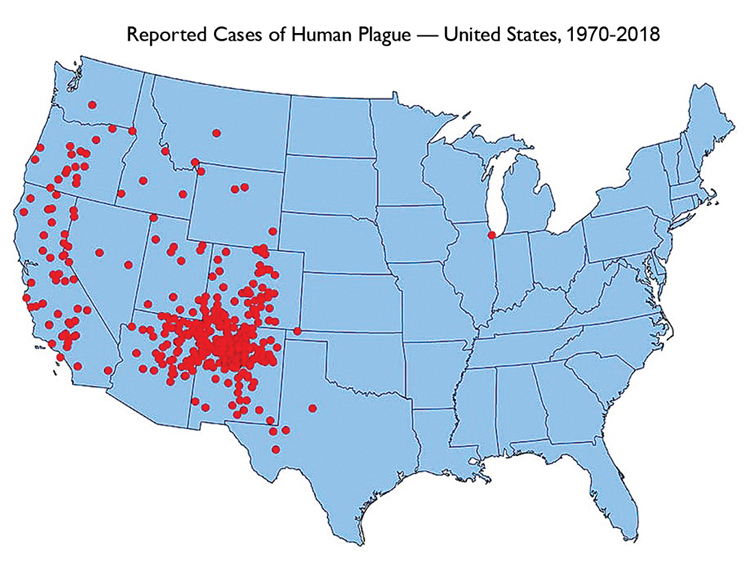
Fig.2. Red dots represent most likely county of exposure for each confirmed plague case. Since the mid-20th century, plague in the United States has typically occurred in the rural West.
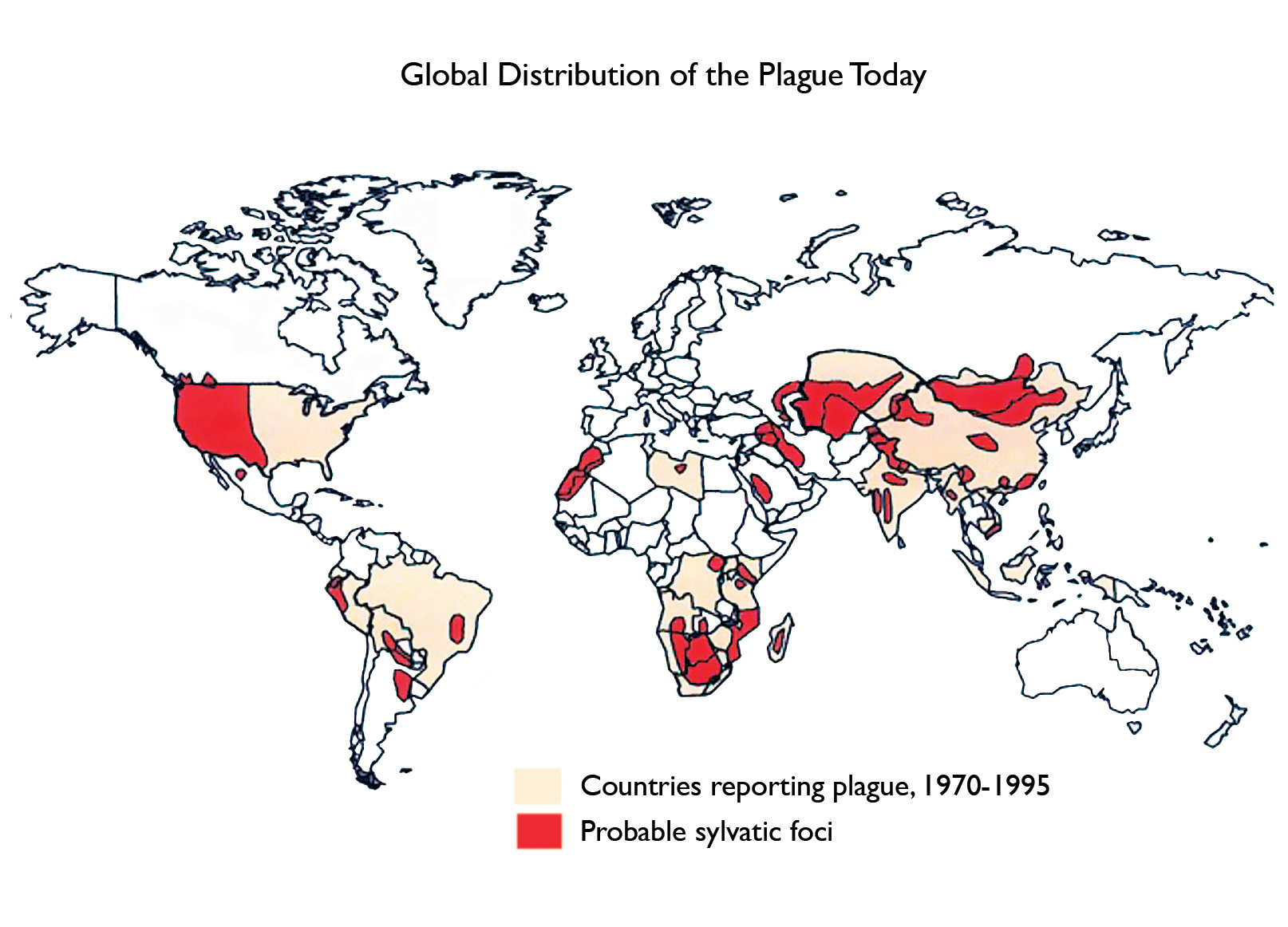
Fig.3. Compiled from the World Health Organization, CDC, and other sources.
MICROBIOLOGY OF BUBONIC PLAGUE
The drastic decline in the incidence of plague since the onset of the third pandemic is largely due to the discovery (near the end of the 19th century) of its causative organism (Yersinia pestis), transmission vector (the flea), and primary reservoir (flea-infected rodents). This knowledge has prompted the implementation of effective public health efforts.
THE CAUSATIVE ORGANISM
Yersinia pestis (formerly known as Bacillus pestis, then Pasteurella pestis) is a non-motile, facultatively anaerobic, intracellular, gram-negative member of the Enterobacteriaceae family.6,7 It has only one phage type, one serotype, and 3 biovars, each of which was responsible for one pandemic—Antiqua for the first, Medievalis for the second, and Orientalis for the third.3 New Orientalis ribotypes have been identified recently in Madagascar, Vietnam, and India. These, together with a burst of phenotypic and genetic diversity, raise concern about selective advantages that could make the plague difficult to control in the future.8
Most Y. pestis virulence factors are encoded on three genus-specific plasmids, the products of which are variably expressed among the different Yersinia species and at different growth temperatures. These include proteins, which inhibit phagocytosis and release of pro-inflammatory cytokines, as well as those which induce apoptosis of macrophages. Other virulence factors include secreted toxins and a plasminogen activator, which promotes spread throughout the body.6
TRANSMISSION VECTORS
Y. pestis infects fleas, most commonly Xenopsylla cheopis (the rat flea), as well as ticks and human lice. Although the plague is commonly associated with rats, more than 200 animal species can serve as hosts, including burrowing rodents (ground squirrels, prairie dogs, marmots, and Asian steppe-dwelling tarabagans), camels, and sheep.4
Surprisingly, most carnivores are highly resistant to Y pestis, with the important exception of the domestic cat which, when infected, can transmit plague to owners or veterinary care givers by direct pneumonic transmission.9 Y. pestis has a fairly broad temperature range. It grows well at 20-28C (68-82F) in fleas, and may remain dormant but viable at lower temperatures. The most recent proposal from the group in Marseille is that it is a telluric organism, i.e. a soil organism, but with an unknown reservoir.10
CLINICAL PRESENTATION
Plague appears in 3 clinical forms: bubonic, septicemic, and pneumonic. Bubonic plague develops after a bite by an infected flea, which introduces Y. pestis into the dermis from which it travels to regional lymph nodes and then to the liver and spleen, where it multiplies very aggressively.3,6 After a 2- to 6-day incubation period, the patient experiences a sudden onset of malaise, headache, shaking chills, and fever. Lymph nodes, most commonly in the groin, axilla, and neck, become inflamed, swollen, and can sometimes reach up to 10 cm (Fig. 4a). These buboes (from which the term bubonic is derived) cause great pain and can rupture percutaneously. Without immediate treatment, 40% to 70% of infected individuals will die.5
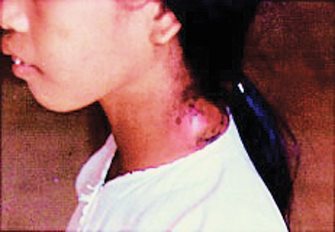
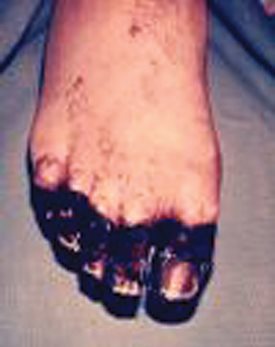
Fig.4a (above): Common lesionsin plague. Patient with
bubo (enlarged, inflammed lymph node) on the neck.
Fig. 4b (left): Gangrene-induced necrosis
on the toes of a patient with plague.
Septicemic plague, which comprises 10% to 15% of all cases, develops either as a primary condition or secondary to bubonic plague. It begins as a massive bacteremia that progresses rapidly to septic shock and disseminated intravascular coagulopathy, often resulting in thromboembolic peripheral gangrene (Fig. 4b). Generalized lymphadenopathy may develop secondarily, along with splenic or hepatic abscesses, endophthalmitis, meningitis, and pneumonia. Without immediate treatment, the mortality is almost 100%; with treatment, it can still reach 40%. 5/p>
Pneumonic plague may also be primary or secondary. In an otherwise healthy individual, primary pneumonic plague begins with the sudden onset of infectious pneumonitis within 24 to 48 hours of exposure. The infection progresses so rapidly that the patient usually dies before it can reach an advanced stage.3 Death is almost certain if treatment does not begin within 18 to 24 hours.4 Secondary pneumonic plague develops when Y. pestis travels hematogenously from an established infection to the lungs, causing severe bronchopneumonia. When it invades the alveoli, Y. pestis can spread by droplet transmission during close contact (≤ 2 yards) with infected individuals, especially in a cold, humid climate.
PREDISPOSING CONDITIONS
It's tempting to say that the 14th century pandemic started with a change in the weather.11 Toward the end of the 10th century Europe experienced a period of global warming that lasted well into the 12th century. Accompanied by improved agricultural technology, crops grew in abundance, contributing to considerable improvements in the health and nutritional status of the average European.2 Between 1000 and 1200 C.E., Europe's population tripled to more than 75 million.1
The explosion of the human population led to increased demand for goods and a surge in trade. Some had already acquired a taste for goods from the Far East through their transactions with merchants in the Middle East. The journey was arduous (2 years by ship to China), and Venice and Genoa wanted their part of the action. The unification of Asia by the Mongols dramatically changed the length and nature of that journey. By 1279 the Mongol Empire was the largest contiguous empire in world history, covering 22% of the earth's total land area and controlling a population of more than 100 million.
At first overland trade traversed a variety of southern routes through or around the Himalayas (The Silk Roads). By 1300 C.E., an easier route was created over relatively flat terrain through the northern steppes of Central Asia. Unfortunately, it took travelers through the territory of an animal enemy much more deadly than Mongols or bandits: the tarabagan. To steppe dwellers, this squirrel-like creature was a flea-infested death trap known for transmitting a deadly respiratory disease. To European traders, it was a valuable source of fur, and being unfamiliar with signs of illness in these animals, traders may have inadvertently transported fleas or flea-infested furs to Europe. The black rat (Rattus rattus) eventually became the primary carrier of Y. pestis-infected fleas. This social, agile creature was well adapted to life in ships' galleys, making it a perfect vector for seaborne transmission of Y. pestis from one Mediterranean port to another, then “jumping ship” to spread the plague throughout these crowded urban centers.
Y. pestis arrived during a tumultuous time in European history. By the end of the 13th century, the economy was under extreme stress. The population surge that resulted from the temporary agricultural abundance of the 11th and 12th centuries had caused overcrowding and fierce competition for food, which was worsened by the arrival of a Little Ice Age in 1300. The cool, wet summers yielded mediocre harvests, and torrential downpours washed away the topsoil. Impoverished peasants abandoned their farms and mobbed the cities, making food scarcer for everyone. Dwellings were largely made of mud, wattle, and thatch, which provided ideal habitats for rats and other vermin. Man and beast were brought even closer together as humans filled the streets with garbage, waste, and dead livestock on which the rats could dine.
Economic instability was soon joined by political calamity, following the introduction of gunpowder and the cannon in 1327 C.E. Longstanding rivalries of the Papacy vs. the Holy Roman Empire, Genoa vs. Venice, Naples vs. Hungary, and France vs. England, exploded into bloody confrontations. In this morbid environment, the plague struck.
When the major pandemic ended around 1351 C.E., it left behind a devastated Europe. The average lifespan decreased from 35-40 years in the 13th century to less than 20 years in the 14th century. The lack of strong young workers left Europe's infrastructure and economy in shambles. Ironically, it also resulted in socioeconomic changes that had long-lasting positive effects:
1. Redistribution of wealth and the end of feudalism:
War, famine, and most natural disasters reduce population and food at the same rate, whereas the plague reduced the population dramatically but did not affect goods or property. Combined with continued economic recession, the decreased demand for food lowered food prices and deprived landlords of capital, while the marked labor shortage enabled peasants to demand higher wages, which they could save to buy land from the upper class. Threatened by this turn of events, landlords attempted to hold onto their assets and power by freezing wages, making it illegal for peasants to refuse work, and subjecting peasants to excessive taxes in an attempt to prevent them from accumulating wealth. The resulting peasant insurrections eventually forced landlords to improve wages and work conditions, thereby bringing an end to the feudal system.
2. Technological advances:
The labor shortage led to the invention of time and labor saving devices in several industries, including book production (e.g. the Gutenberg press), mining (e.g. water pumps), and war (improved weaponry).
3. Science and medicine:
The Plague advanced the practice of medicine from magic and superstition, including Galenic medicine (balancing the “four humors” and avoiding “bad air”) to techniques that formed the basis of modern medicine. Those physicians who did not abandon their communities developed practical, clinically oriented medical procedures to streamline patient care and improve hospital procedures with the goal of achieving a cure rather than merely giving patients a place to die. One example is development of the ward system, in which patients with the same illness were housed together to facilitate their care. Scholarly activities resulted in more accurate textbooks and the development of the scientific method. They also generated new ideas about the spread of disease that evolved into the first systematic theory of contagion.
4. Public health:
Two institutions were created to prevent transmission of the plague: the municipal health board, which oversaw sanitation and burial of the dead; and the plague house — part hospital, part rehabilitation unit, and part prison – which became the first institution to use quarantine * to prevent the spread of disease.
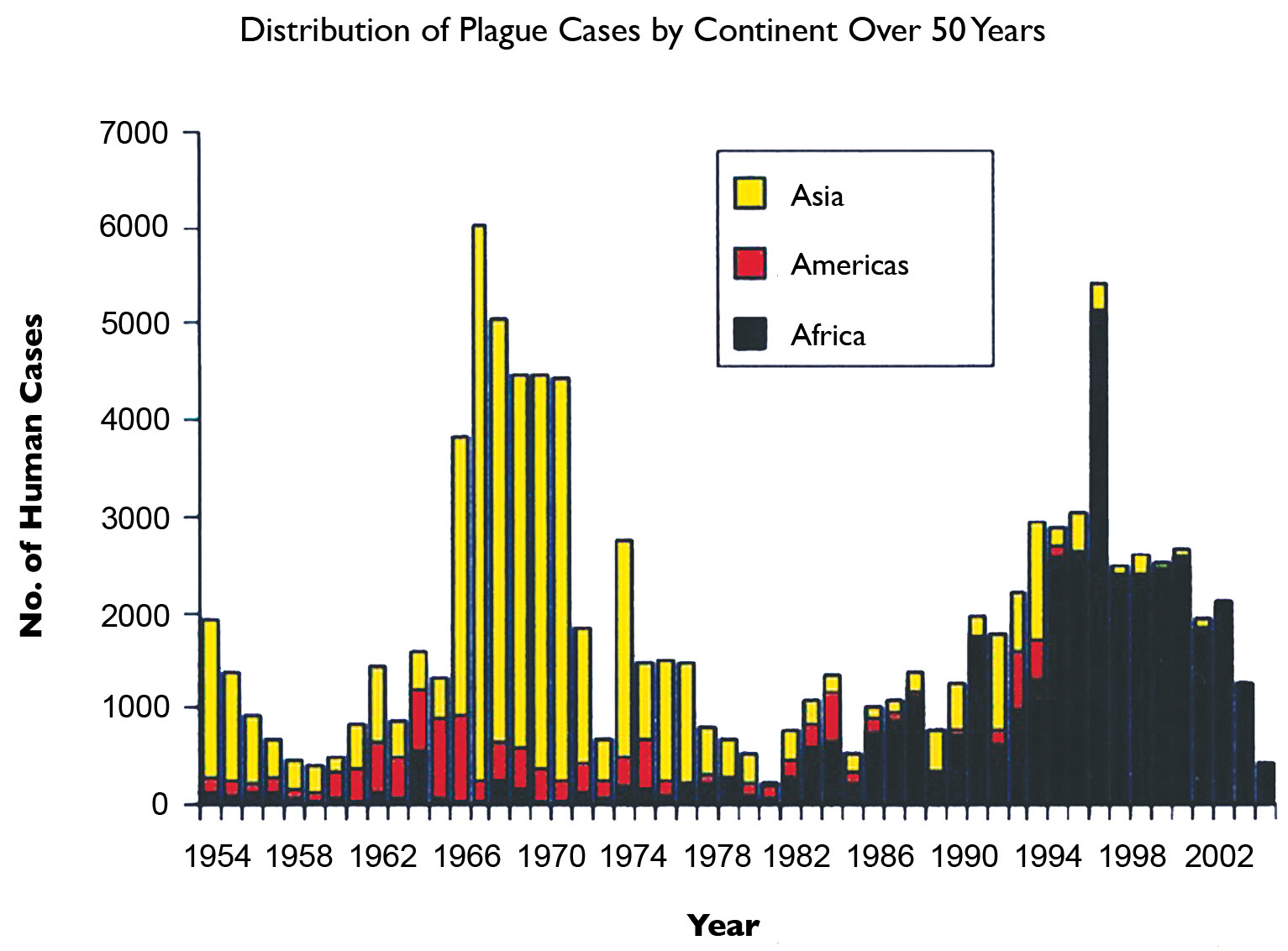
Fig.5. Distribution of plague cases by continent over 5 decades.
LESSONS LEARNED
The Plague Is Still with Us
Today, Bubonic Plague remains a threat in many parts of the world, albeit with continuously changing demographics (Fig. 5) and a much more complex transmission pattern than was previously thought. Of additional concern is recent evidence that multi-drug resistance can be conferred to Y. pestis by plasmids from other Enterobacteriaceae, including reports of a multiple-resistant strain identified in Madagascar as early as 1995.12 Moreover, many of the conditions that facilitated the spread of bubonic plague during the 14th century persist to some extent today, in addition to some uniquely modern influences.
Global warming trends indicate that throughout the 21st century certain geographic regions will experience decreased precipitation and droughts, while others will see an increase in precipitation and floods. In high-risk areas of the world such changes can compromise food and water quality, while increasing the prevalence and severity of disease in both human and rodent communities.
International travel allows the spread of infection on a scale and with a speed never possible before.
Political unrest and population displacement in large areas of Africa and the Middle East make it difficult to control factors that foster the persistence of plague, including poverty, malnutrition, chronic infections (especially tuberculosis, HIV, and malaria), and inadequate health care.
BIOTERRORISM
There is significant concern over the potential of plague to be used as a biological weapon. In 1347 the Tartars catapulted bodies of plague victims over city walls during a siege, and in World War II the Japanese dropped bombs containing fleas inoculated with Y pestis. During the Cold War, the Soviets succeeded in aerosolizing the bacteria and in creating strains of multidrug-resistant Yersinia. Aerosolized Y pestis, which causes pneumonic plague, has one of the highest potentials as a bioterrorism agent, and has been classified as a Category A, or high priority, bioterrorism agent by the CDC.
BRINGING THE THIRD PANDEMIC TO A CLOSE
If nothing else, the Plague pandemic of the 14th century teaches us to be vigilant against key plague-promoting factors, including changes in Y. pestis demographics, transmission factors, and climate.11 Advances in immunology and genomics allow us to monitor the causative organism for the development of drug-resistance and to facilitate the selection of the most effective antibiotics.13 The rare incidences of immunity that offered hope to 14th century survivors reinforce the need for effective vaccines. The “traditional” anti-plague vaccines are based on a live attenuated strain and a killed whole-cell preparation, and do not take into account strain-related differences in virulence, nor do they protect against the deadly pneumonic form of the disease. Researchers are now investigating strain-specific subunit vaccines, which seem very promising.
History also teaches us the importance of public health education that promotes preventive practices and timely treatment, especially in the most vulnerable areas of the world. These observations highlight the importance of maintaining a worldwide system for reporting disease outbreaks.
Lessons from SARS
In 1997, WHO developed the Global Outbreak Alert and Response Network (GOARN), which annually identifies and responds to at least 50 outbreaks of various infectious diseases through partner networks in more than 120 countries.14 GOARN was first tested during the Severe Acute Respiratory Syndrome (SARS) outbreak which began in China. It was able to coordinate a global response that promoted heightened vigilance and linked laboratory scientists, clinicians, and epidemiologists from around the world in a virtual network to rapidly identify the causative agent, its mode of transmission, and its pertinent epidemiologic factors. WHO was thus able to guide health care workers in developing effective procedures for clinical management and protection that curtailed the nosocomial spread of this disease, while issuing warnings for international travelers. Within 4 months after this system received accurate information, SARS transmission had been interrupted in all partner sites.14
The SARS experience revealed several potential barriers to GOARN’s effectiveness, including the following:
1. Difficulty obtaining timely, accurate, and complete information: Initially The People's Republic of China perpetrated a cover-up and did not indicate the true number of cases and their location. This resulted in the secondary spread of SARS to health care workers and then throughout China and the rest of the world;
2. Inadequacy of translation services: GOARN's Canadian partner—the Global Public Health Intelligence Network (GPHIN)—identified a “flu epidemic” in China while monitoring the internet. GPHIN had the facility to translate reports into several languages, including Chinese, but was only able to transmit information to GOARN in English and French. This delayed the transmission of pertinent information to the Chinese government, which allowed the continued spread of this infection.
Hopefully these experiences have made most nations aware of the importance of providing accurate and complete information as soon as an outbreak of a communicable disease is suspected.
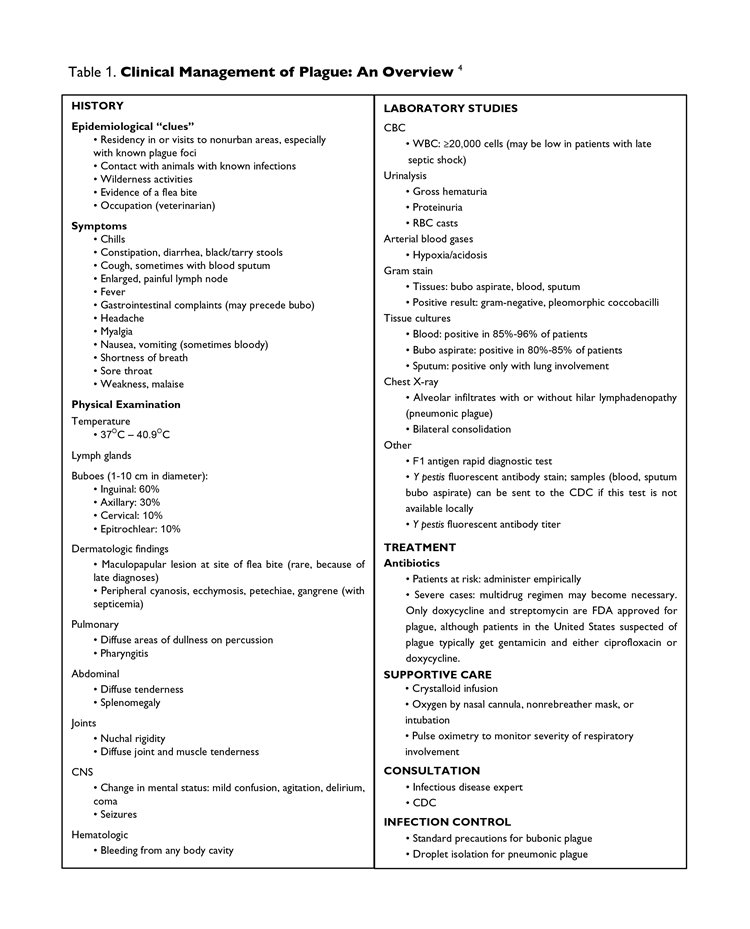
Most importantly, the effectiveness of any containment efforts begins with primary health care providers who are prepared to provide infection-specific care to their patients and promote disease prevention. Unfortunately, there is considerable room for improvement in physician training for plague management (Table 1). As of 2004, only 28% of U.S. office-based physicians were trained specifically in this issue.15 Because patients with the bubonic form of the disease may present to their physician with flu-like symptoms and adenopathy, office-based physicians must know when to suspect plague, how to confirm their suspicions, how to treat such patients, and when to refer them to an infectious disease specialist. Patients with septicemia or the pneumonic form of the disease are more likely to be seen in the EMD or the morgue.
CONCLUSIONS
The incidence of plague today may seem miniscule compared with the 14th century. Still, its reappearance in the 20th and 21st century mandates a well-planned strategy for preventing new outbreaks. Information is our greatest weapon against this disease. By monitoring potential contributing factors, educating the public, improving physician training, and developing strain-specific vaccines, we may be able to reduce the number and size of plague loci and prevent the plague-related devastations of the past from ever recurring.
* The word quarantine is derived from an amalgam of the Italian words quaranta giorni, or 40 days. Ships arriving in Venice were required to wait at anchor 40 days before landing.
REFERENCES
1. Potok C. Wanderings: Chaim Potok's History of the Jews. New York: Ballantine Books; 1978: 425.
2. Kelly J. The Great Mortality: an Intimate History of the Black Death, the Most Devastating Plague of All Time. New York: HarperCollins; 2005.
3. Riedel S. Plague: from natural disease to bioterrorism. Baylor U Med Center Proc. 2005;18: 116-124. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1200711/
4. Velendzas DG. CBRNE: Plague. https://emedicine.medscape.com/article/829233-overview
5. Stenseth NC, Atshabar BB, Begon M, et al. Plague: past, present, and future. PLoS Medicine. 2008;5:0009-0013. https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.0050003
6. Huang X-Z, Nikolich MP, Lindler LE. Current trends in plague research: from genomics to virulence. Clin Med & Res. 2006; 4:189-199.
7. Perry RD, Fetherston JD. Yersinia pestis: etiologic agent of plague. Clin Microbiol Rev. 1997;10;35-66.
8. Anisimov, AP, Lindler LE, Pier GB. Intraspecific diversity of Yersinia pestis. Clin Microbiol Rev. 2004; 17:434-464.
9. Gage, KL et al. Cases of cat-associated human plague in the Western US: 1977-1998. Clin Inf Dis. 2000;30;893-900.
10. Drancourt M, et. al. Yersinia pestis as a telluric, human ectoparasite-borne organism. Lancet Inf Dis. 2006;6: 234-241
11. US Environmental Protection Agency. Climate Change-Science. Future Precipitation and Storm Changes. https://19january2017snapshot.epa.gov/climate-change-science/future-climate-change_.html
12. Galimand M, Guioyoule A, Gerbaud G, et. al. Multidrug resistance in Yersinia pestis mediated by a transferable plasmid. New Eng J Med. 1997;337 :677-680.
13. Glasner JD, Plunket II G, Anderson BD, et al. Enteropathogen Resource Integration Center (ERIC): bioinformatics support for research on biodefense-relevant enterobacteria. Nucl Acids Res. 2008;36: D519-D523.
14. Heymann DL, Rodier G. Global surveillance, national surveillance, and SARS. Emerg Infect Dis. 2004;10: 173-175. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3322938/
15. Niska RW, Burt CW. National Ambulatory Medical Care Survey: terrorism preparedness among office-based physicians, United States, 2003-2004. Advance data from vital and health statistics; no 380. Hyattsville, MD: National Center for Health Statistics. 2006. https://pubmed.ncbi.nlm.nih.gov/17702147/