Spring 2020 - Vol. 15, No. 1

Advancement of Therapy for Glioblastoma
Kristine Dziurzynski, M.D.
Penn Medicine Lancaster General Health Physicians
NeuroScience & Spine Associates
INTRODUCTION
Over the past decade, there has been an evolution in the standard of care for patients with glioblastomas, as for most malignancies, resulting in tangible benefits in survival and quality of life. This article will be the first in a series of four:
1. An overview of advances in glioblastoma therapy;
2. Review of immunotherapy for glioblastoma;
3. Advances in surgical technology and approach;
4. Application of these advances at Lancaster General Hospital.
ADVANCEMENT OF GLIOBLASTOMA THERAPY
Current Standard of Care and State of the Disease
The standard of care for newly diagnosed glioblastomas consists of three phases. First, surgical resection of the tumor to the maximum, safe extent possible. Next, chemotherapy with temozolomide concurrently with radiation. Finally, administration of temozolomide for an additional (adjuvant) six cycles, 5 of every 28 days, with the addition of tumor treating fields (TTF)
1
 Fig 1. This image demonstrates the mobility of a patient using the Novocure Optune tumor treating fields device (TTF), and how it is worn. Patients can conceal the device with various types of headwear.
Fig 1. This image demonstrates the mobility of a patient using the Novocure Optune tumor treating fields device (TTF), and how it is worn. Patients can conceal the device with various types of headwear.
Tumor treating fields (TTF) is a device worn on the scalp at least 18 hours per day to deliver a small electric current that disrupts microtubule assembly, inhibiting mitosis in glioblastoma cells (Fig. 1). The study that identified the benefit of TTF was the first study since the addition of temozolomide to radiation in 2005 that showed a statistically significant benefit in a phase 3 clinical trial. Nonetheless, its use has not been widely adopted.
The TTF study was criticized both for not including a placebo device, and for the effect attributed to the increased attention patients receive when enrolled in a clinical trial. Proponents for TTF have responded in kind that some of these same criticisms apply to the 2005 temozolomide study. At the time of tumor recurrence, treatment is less uniform, and may consist of any of the following as appropriate to the patient: clinical trial, additional chemotherapy, re-irradiation, TTF.
Median overall survival of patients with glioblastoma remains officially quoted as 14 months, a number generated from study results in 2005.2 For patients enrolled in the treatment arm of a clinical trial, median survival has increased up to 16 months,3 and there are anecdotal reports of median survival rates up to 18-22 months for patients enrolled in a clinical trial at a large, renowned cancer center. Notably, these favorable statistics have been criticized for selection bias, as patients tend to be younger, and less debilitated by their disease.
The most important and objective finding that has emerged over the past 10 years to explain why some patients with glioblastoma live longer and perform better than others, is the ability to identify the molecular profile of an individual patient’s tumor.
The underlying principles of molecular profiling apply to patients in general, and shape the search for a cure, so this review of advances in glioblastoma care will lead with these new discoveries on the molecular front.
ADVANCES IN GENOMIC DRIVERS OF DISEASE AND IN PATHOLOGIC DIAGNOSIS
Today, for all types of cancer, understanding an individual tumor’s genome is fundamental to understanding the disease’s behavior and treatment. The discovery of multiple genes and their mutations has allowed more accurate characterization of each type of cancer; of better understanding of its behavior; how and why some cancers with the same histology behave more aggressively than others; and which ones will respond to treatment. This understanding is the current foundation of cancer care.
For glioblastomas, multiple mutated genes have been identified as drivers of the disease.4,5 The genomic alterations of MGMT (O6-methylguanine-DNA-methyltransferase) methylation, IDH (isocitrate dehydrogenase) mutation, and 1p19q co-deletion are highlighted here. The relevance of 1p19q will be exemplified from a related malignant glioma. These are examples of how mutations govern tumor behavior and response to treatment.6,7,8,9
In the same issue of the New England Journal of Medicine that contained the findings leading to the addition of temozolomide as the standard of care, the significance of MGMT methylation was reported as a companion study.6 MGMT is a DNA repair enzyme, and temozolomide works by damaging tumor DNA through the addition of a methyl group to one of its nucleic acids. If MGMT happens to be turned off, patients respond better to the chemotherapy. This phenomenon is epigenetic, whereby the promoter for the MGMT gene happens to be methylated itself. Testing for MGMT promoter methylation is the standard of care for GBM patients, is critical for prognostication, and is now included in all pathology reports.5
In 2005, the Cancer Genome Atlas (TCGA) Research Network was established, tasked “with the aim of obtaining a comprehensive understanding of the genomic alterations that underlie all major cancers.” Lung, brain and ovarian cancers were the first ones selected for genome mapping in 2006.10 Separately, in 2008, IDH mutation was found to occur in 12% of glioblastoma patients, associated with a very strong increase in survival. Median survival in patients harboring an IDH mutation was 3.7 years compared to an average of 1.1 years in others.7 IDH is an enzyme in the Krebs Cycle that converts isocitrate to α-ketoglutarate, and NAD+ to NADH. Prior to the discovery of the mutation, there was no known association of IDH with cancer. The mutated protein converts α-ketoglutarate to 2-hydroxyglutarate (2-HG), which has been found to be elevated in IDH-mutated gliomas.11 These findings led to the hypothesis that mutant IDH is an oncogene and 2-HG is an oncometabolite.12
One of the first reports of The Cancer Genome Atlas (TCGA) for glioblastoma was issued in 2010. Based on gene clustering data, it described four subtypes of glioblastoma, and correlated them with the responses to treatment.8 The gene that encodes IDH mutation was one of two genes that defined the proneural subtype, which was associated with the most favorable prognosis.
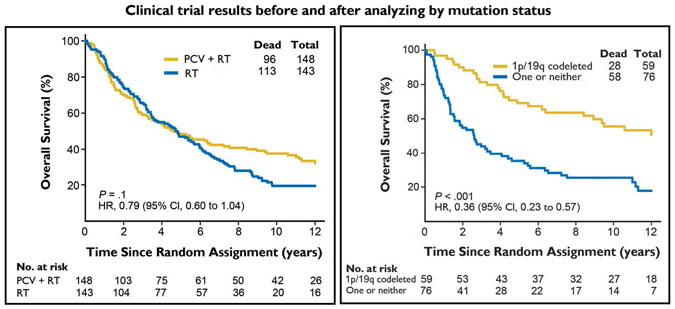
Fig. 2 (left). Kaplan-Meier estimates of overall survival by treatment group. For patients treated with procarbazine, lomustine, and vincristine (PCV) plus radiotherapy (RT), compared with RT alone, the hazard ratio (HR 0.79) was not significantly different (95% CI, 0.60 to 1.04; P = .1).
Fig 3 (right). Kaplan-Meier estimates of overall survival by genotype for procarbazine, lomustine, and vincristine plus radiotherapy arm. For patients with 1p/19q codeleted anaplastic oligodendroglioma (AO)/anaplastic oligoastrocytoma (AOA) compared with those with AO/AOA in whom one or neither allele was deleted, the hazard ratio (HR 0.36) was significantly better (95% CI, 0.23 to 0.57; P < .001).
The impact of specific mutations on response to treatment became very apparent in 2012, when the results of a large phase 3 clinical trial of malignant oligodendrogliomas, a less aggressive tumor in the same class as glioblastoma, were re-analyzed based upon mutation status. When study results had first been reported in 2006, all diagnoses were made histologically, and there were no differences in survival between treatment groups 13 (Fig. 2). However, after tissues were re-analyzed in light of recent mutational discoveries, and patients’ diagnoses were re-stratified based upon molecular markers, there were striking differences for patients with favorable markers. Those with MGMT methylation, Ip19q co-deletion had median survival rates of 14.7 years, vs. 2.6 years for those with no deletions (Fig. 3). 9 Co-deletion of the 1p19q chromosome is the signature diagnostic mutation that defines an oligodendroglioma. Importantly, all 1p19q co-deleted tumors are IDH mutated.14
The World Health Organization is the official international gold standard for determining the diagnosis of brain tumors. Its pathologic criteria were updated in 2016 to mandate the incorporation of molecular markers that have been vetted as reliable qualifiers of disease; histology alone is no longer sufficient for diagnosis.5
ADVANCES IN SURGICAL TECHNOLOGY
The first step in the treatment of glioblastoma is surgical resection, and the current standard is performance of a maximal safe resection.15 The degree of surgical aggressiveness depends on the tumor’s location.16,17 If the tumor is in or near an eloquent region of brain, i.e. one that governs a function such as language, movement, or vision, adjuncts can be used to reduce surgical risk and improve safety.
Intraoperative navigation using GPS technology has been in routine use since the 1990s to synchronize with the MRI where a person’s tumor is in space. Plus, in the past decade we have acquired the ability to demonstrate critical white matter tracts in color on the MRI, and to use this technology intraoperatively for surgical planning (Fig. 4). This information enables the surgeon to assess the tumor’s involvement of critical structures, and how best to avoid damaging them when approaching, dissecting, or removing the tumor.
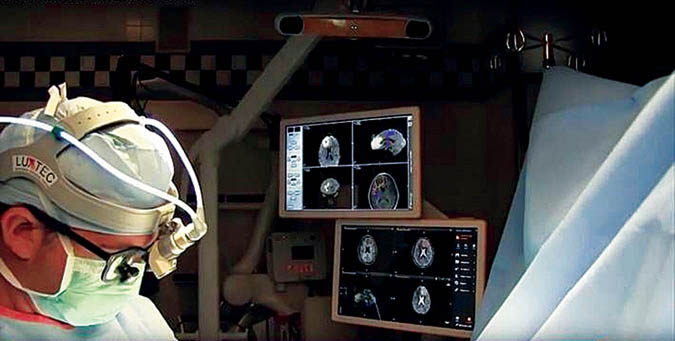
Fig. 4. Identification of critical white matter tracts in color on the MRI can be used intraoperatively for surgical planning.
Awake craniotomy, which has been utilized for decades, is a technique in which eligible patients are awakened after the tumor has been exposed, and can be asked to perform tasks governed by the areas of brain involved with tumor. To determine which areas can safely be removed, the region in question is mapped using a probe that emits a small electric current. If this stimulus arrests speech or motor function, the risk of removal is prohibitive, and that portion of brain or tumor must be left behind.
Other brain mapping techniques exploit our ability to detect electric potentials of the brain that establish distinct signatures. These can be performed with patients asleep to map out those gyri that are part of eloquent anatomy.
As of 2017, the FDA has approved intraoperative use of 5-ALA, a heme metabolite that renders tumor cells a fluorescent red under the intra-operative microscope when subjected to filtered light. It has a high degree of specificity and has been shown to double the rate of gross total resection.18
ADVANCES IN THERAPEUTICS
Precision Medicine
In cancer, this term is officially defined as “specific information about a person’s tumor to help diagnose, plan treatment, find out how well treatment is working, or make a prognosis.” It is based upon targeted therapy, and in oncology it involves treatments that are targeted to specific molecular drivers of the cancer.
For glioblastoma multiforme, there has been limited progress in this arena, due to the highly variable molecular characteristics of these tumors (“multiforme” is an apt qualifier in its formal surname). Although many mutations have been identified, they are not uniformly expressed throughout the cells of a glioblastoma, not even in each individual tumor. Thus, no single agent that will substantially prolong survival is envisioned as the future of therapy for all these patients. Multiple modalities of treatment are expected to emerge, much as the right tool or tools from a toolbox might be needed to get the job done.
Precision medicine is built upon identifying the mutations per patient per tumor, and selecting the drugs or therapies that specifically treat those identified. For the reasons cited above, few such therapies exist for glioblastoma. One reason is that the function of certain mutated genes has not been identified. Another is the challenge of developing drugs that can penetrate the blood brain barrier, which most current chemotherapy agents cannot do. The therapeutic targets of current active research are summarized in Fig. 5.
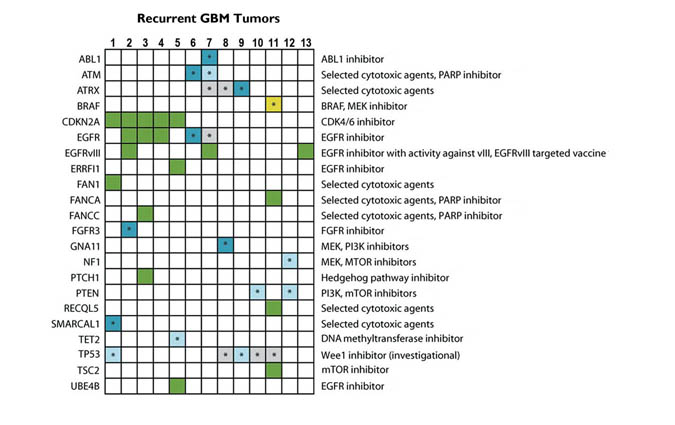 Fig. 5. Potential therapeutically actionable alterations identified in recurrent glioblastoma samples.
Fig. 5. Potential therapeutically actionable alterations identified in recurrent glioblastoma samples.
Immunotherapy
Immunotherapy has recently been the focus of increased media attention, largely due to success in treating other malignancies such as leukemia, melanoma, and lung cancer, where there are reports of some patient cohorts experiencing remissions for 3-5 years.19,20,21 For glioblastoma, there have been early reports at meetings of clinical trials of immunotherapy that identified a group of responders with long-term survival up to 5 years. Even though this degree of benefit was not realized among the larger cohort, the results among the responders are so significant that this modality remains a viable option, and robust research efforts continue.
Multiple therapeutic modalities fall under the umbrella of immunotherapy. These include different types of vaccines; engineered T cells (CAR T); and immune checkpoint inhibitors. Glioblastoma vaccine types include peptide vaccines, vaccines derived from a patients’ own dendric cells pulsed with their own tumor lysate, and oncolytic viruses.
CAR T therapy involves engineering a T cell to function as a killer cell targeted to the tumor. The patient’s own T cells are removed from their blood to re-engineer a receptor to bind to a specific tumor target. The engineered receptor is referred to as the chimeric antigen receptor (CAR).
Check point inhibitors work by blocking molecular “checkpoints” of an immune response. Under normal physiologic conditions, these checkpoints serve to inhibit T cell activation from developing into an autoimmune response. A cancer will exploit these pathways to prevent the immune system from detecting or attacking the tumor, keeping the immune response either turned off or substantially weakened.22 By inhibiting the checkpoint, immune response against the tumor can be activated. Currently, two inhibitors to checkpoint molecules are being assessed in clinical trials: ipilimumab–Yervoy and nivolumab–Opdivo, respectively, are being evaluated against T-cell surface checkpoint molecules CTLA-4 and PD-1.
These modalities will be explored in more detail in a future article.
Radiation Therapy
Radiation Therapy has been the mainstay of treatment for glioblastoma for several decades. As alluded to earlier, it wasn’t until 2005 that any benefit from the addition of chemotherapy was identified. The current standard dose of 60 Gy (Gray) was established in the 1970s. Subsequent studies identified a dose relationship to tumoricidal activity, but no additional benefit beyond the dose of 60 Gy.23,24 To place this dose into context, whole brain radiotherapy to treat brain metastases is dosed at 30 Gy, stereotactic radiosurgery to treat isolated brain metastases uses 18-24 Gy. But despite the high dose used for treatment, most glioblastomas reoccur within the region of the treated tumor.
With improvements in technology, such as IMRT (Intensity Modulated Radiation Therapy),25 more of the dose can be concentrated within the treated tumor volume, meaning the treatment is more conformal and reduces exposure of surrounding brain tissue. This technique not only improves safety and quality of life, but has been shown to improve survival in glioblastoma.26 Another technical advance in IMRT is the development of multi-leaf collimators, which better control the direction and focus of the beam, and further improve conformality and precise dose delivery to the tumor.
The development of improved collimators has contributed to renewal of interest in proton therapy, which was first proposed in 1946, and first used in patients in 1958. Whereas traditional radiation therapy uses photon beams which are waves of energy with no mass, proton therapy uses an accelerated charged particle, the proton. These are usually generated by separating a hydrogen atom’s electron, advancing it through an electric field, and accelerating it with a cyclotron. From there, it is passed through multiple devices, including the collimator, prior to delivery to the patient’s tumor (Figs. 6 and. 7).
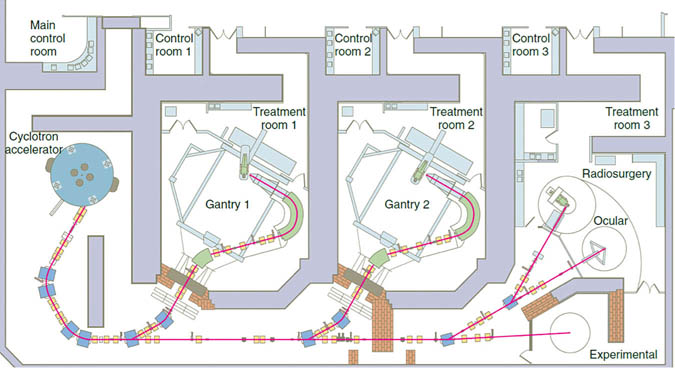
Fig. 6. This schematic of Harvard’s proton beam center shows the multiple devices used to improve conformality of the beam prior to the gantry, which also is fitted with additional devices to more precisely cover the tumor. https://gray.mgh.harvard.edu/clinical-physics
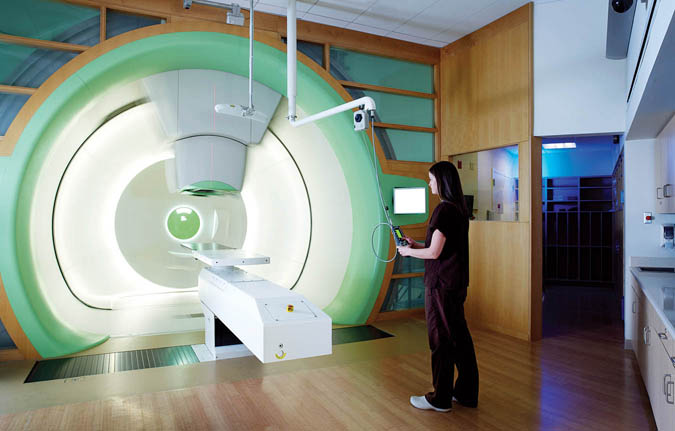
Fig. 7. In this photo of the treatment room at the University of Pennsylvania’s proton center, the massive amount of machinery that delivers the beam to the patient is discreetly hidden behind the wall. https://www.pennmedicine.org/cancer/navigating-cancer-care/treatment-types/proton-therapy
The use of a physical entity, the proton, results in more of the maximal dose remaining in the tumor, unlike photon beam therapy where more of the energy spreads into surrounding tissue. The result is a dramatic difference in the exposure of surrounding brain tissue to the “radiation bath” (Fig. 8). Additionally, a recent Penn study comparing patients under treatment with proton and concurrent chemotherapy compared with patients under treatment with photon and concurrent chemotherapy, showed a two-thirds reduction in relative risk of 90-day grade 3 toxicity requiring hospitalization in a range of cancers, including brain tumors.27 In pediatric brain tumors, compared with photon therapy, proton treatment reduces the radiation dose to surrounding brain tissue, diminishing acute toxicities without compromising disease control.28 Currently, an international trial is underway to evaluate proton therapy in adult brain tumors.
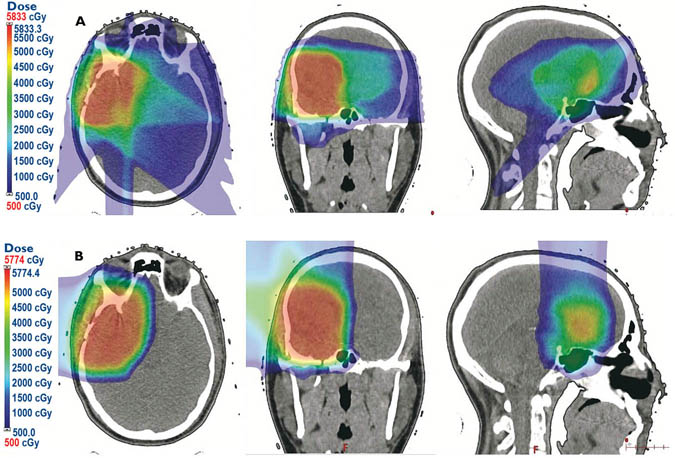
Fig. 8: A. Conventional IMRT photon beam therapy plan for a low-grade astrocytoma of the right frontal lobe; B. A PBS-PT (Pencil Beam Scanning Proton Therapy) plan for the same tumor.
CONCLUSIONS
Advancement of care for glioblastoma is rooted in general molecular biology and technology. For all cancers, the identification of specific molecular drivers of disease, coupled with more precise mechanisms to deliver treatment, are projected to result in safer, more efficacious, and more durable responses. Although this promise of better outcomes is in an early phase of development, preliminary results have greatly energized the clinical and research communities, who are committed to transforming their work into long term survival for patients.
The next article in this series will provide a deeper overview of progress and active research efforts in immunotherapy for glioblastoma.
REFERENCES
1. National Comprehensive Cancer Network. Guidelines for Central Nervous System Malignancies. https://doi.org/10.6004/jnccn.2017.0166
2. Stupp R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352:987-996.
3. Shahar T, Nossek E, et al. The impact of enrollment in clinical trials on survival of patients with glioblastoma. J Clin Neurosci. 2012; 19:1530-1534.
4. The Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2013; 494:1061-1068.
5. Louis DN, Perry A, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016; 131:803-820.
6. Hegi ME, et al. MGMT Gene Silencing and Benefit from Temozolomide in Glioblastoma. N Engl J Med. 2005; 352:997-1003.
7. Parsons DW, Jones S, Zhang X, et al. An Integrated Genomic Analysis of Human Glioblastoma Multiforme. Science. 2008; 321:1807-1812.
8. Verhaak Roel GW, Hoadley KA, Purdom E, et al. An integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR and NF1. Cancer Cell. 2010; 19:98-110.
9. Carincross G, Wang M, Shaw E, et al. Phase III Trial of Chemoradiotherapy for Anaplastic Oligodendroglioma: Long-term Results of RTOG 9402. J Clin Oncol. 2013; 31:337-343.
10. https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga/history/timeline
11. Dang l, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009; 462 (7274):739-44.
12. Garber K. Oncometabolite? IDH1 discoveries raise possibility of new metabolism targets in brain cancers and leukemia. J Natl Cancer Inst. 2010; 102 (13): 926-8.
13. Carincross G, Berkey B, Shaw E, et al. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol. 2006;2707-2714.
14. Labussière A, Idbaih X, et al. All the 1p19q codeleted gliomas are mutated on IDH1 or IDH2. Neurol. 2010; 74:1886-1890.
15. Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J Neurosurg. 2001; 95:190–198.
16. Duffau H. Is supratotal resection of glioblastoma in noneloquent areas possible? Perspectives. World Neurosurg. 2014; 82: e101-e103.
17. Incekara F, Koene S, et al. Association Between Supratotal Glioblastoma Resection and Patient Survival: A Systematic Review and Meta-Analysis. World Neurosurg. 2019; 127: 617-624.
18. Stummer W, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006; 7:392-401.
19. Schuster SJ, Svoboda J, Chong E, et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N Engl J Med. 2017; 377: 2545-2554.
20. Wolchok JD, et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2017; 377;1345-1356
21. Assi HI, Kamphorst AO, et al. Immune Checkpoint Inhibitors in Advanced Non-Small Cell Lung Cancer. Cancer. 2018; 124:248-261.
22. Lim M, Xia Y, Bettegowda C, Weller M. Current state of immunotherapy for glioblastoma. Nature Rev Clin Oncol. 2018; 15:422-442.
23. Nelson DF, et al. Combined modality approach to treatment of malignant gliomas—re-evaluation of RTOG 7401/ECOG 1374 with long-term follow-up: a joint study of the Radiation Therapy Oncology group and the Eastern Cooperative Oncology Group. NCI Monogr. 1988; 6:279-284.
24. Loeffler JS, et al. Radiosurgery as part of the initial management of patients with malignant gliomas. J Clin Oncol. 1992; 10: 1379-1385.
25. Eshleman JS. A new tool to help fight cancer – tomotherapy. J Lanc Gen Hosp. 2009; 4(3): 106-112.
26. Narayana A, et al. Intensity-modulated radiotherapy in high-grade gliomas: clinical and dosimetric results. Int J Radiat Oncol Biol Phys 2006; 64:892-897.
27. Bauman B, et al. Comparative effectiveness of proton vs photon therapy as part of concurrent chemoradiotherapy for locally advanced cancer. JAMA Oncology 2019, published online December 26, 2019. E1-E10. doi: 10.1001/jamaoncol.2019.4889.
28. Yuan T, Zhan Z, Qian C. New frontiers in proton therapy. Cancer Commun (Lond). 2019; 39(1):61