Winter 2019 - Vol. 14, No. 4
Ton-That Coleman
Is Fibromyalgia Real?
Etiology, Pathophysiology, and Diagnostic Criteria
Tony Ton-That, M.D.
Medical Director Spine/Low Back Pain
Lancaster General Health Physicians Pain Management
Christa Coleman, PsyD, BCB
Clinical Psychologist
Penn Medicine Lancaster General Health Physicians Neuropsychology
First of two parts
INTRODUCTION
Fibromyalgia is a condition characterized by widespread pain and tenderness to touch, and is often associated with disorders related to fatigue, sleep, depression, anxiety, and memory. For many years, a wide variety of painful syndromes has been attributed to a vaguely defined disorder of the muscles, tendons, ligaments, and fascia. Symptoms of chronic backache and neck ache, temporomandibular joint syndrome, and a variety of pelvic, limb and chest wall pains have now been recognized as due to myofascial pain, fibromyalgia (FM), or fibrositis.
Sir William Gowers in 1904 invented the latter term, saying: “I think we need a designation for inflammation of the fibrous tissue. We may conveniently follow the analogy of cellulitis and term it fibrositis.”
1
In 1933 a British Medical Association committee recognized subgroups of fibrositis:
2 intramuscular and fascial; periarticular; bursal and tenosynovial; subcutaneous (panniculitis), and perineuritic. Perineuritis could cause radiating pain, paraesthesias, cutaneous hyperaesthesia, tenderness in muscles and joints within the sensory innervation, and local peripheral nerve tenderness attributed to involvement of the nervi nervorum. Slowly, the concept of the fibrositic nodule arose, an area in the substance of a muscle or its tendinous sheath which causes localized pain, or – when stimulated – referred pain at a distance.
3
It has been clear for some time that patients with fibromyalgia syndrome should not be treated as hypochondriacs just because they manifest psychological symptoms. Chronic pain and tenderness for any reason usually cause psychological distress (see below).
DIAGNOSTIC CRITERIA
A diagnosis of fibromyalgia remains a valid construct irrespective of other diagnoses. The diagnostic criteria for fibromyagia syndrome published in 1990 by the American College of Rheumatology
4 specified patients had to have:
1. Widespread pain in all four quadrants of their body for a minimum of three months;
2. At least 11 of the 18 specific tender points when palpated with 4kg/cm2 of force.
However, in 2010 the American College of Rheumatology published new proposed criteria, which no longer require physical examination for a diagnosis of fibromyalgia. The new diagnostic criteria are based on the presence of three conditions:
1. Widespread pain as evidenced by a Widespread Pain Index (WPI) score of at least 7 and a Symptom Severity (SS) score of 5 or greater; or WPI score 3-6 and SS score of at least 9.
2. Symptoms for at least three months;
3. exclusion of all other disorders which might be expected to account for these symptoms.
PHYSICAL FINDINGS IN FIBROMYALGIA
Fibromyalgia and myofascial pain syndrome are two pain syndromes that may overlap and be difficult to differentiate. Trigger points (TPs) are allegedly an important sign of myofascial pain syndrome and fibromyalgia. Patients with fibromyalgia have tender points, but patients with myofascial pain syndrome may also have trigger points.
A classic TP is defined as the presence of discrete focal tenderness located in a palpable taut band of skeletal muscle, which produces both referred regional pain (zone of reference) and a local twitch response. The mechanism of myofascial pain with characteristic TPs and taut bands is said to be a spinal reflex disorder caused by a reverberating circuit of sustained neural activity in a specific spinal cord segment.
Tender points, by comparison, are associated with pain at the site of palpation only, are not associated with referred pain, and occur in the insertion zone of muscles, not in taut bands in the muscle belly.
High tender point count may indicate more somatic symptoms, more severe fatigue, low level of self-care, increased medical care usage, and high level of distress.
OVERLAPPING CONDITIONS AND DIFFERENTIAL DIAGNOSIS
The initial formation of myofascial Trigger Points may have many diverse precipitants: trauma, muscular strains, fatigued muscle, or nerve root injury. These injuries can activate latent TPs and release neurokinins, histamine, serotonin and prostaglandins, which stimulate nociceptors and cause reflex muscle contraction.
It is prudent to be cautious in diagnosing the precise origin of such pain. Psychogenic, central, and peripheral neural mechanisms may each play a role. Afferents from sites of referred muscle pain are the same as those that act centrally on the spinal neurons that process pain information from deep structures, thus leading to central summation and probable enhancement.
Nerve trunk pain may also simulate myofascial pain and has been recognized in patients with cervical or lumbar radiculopathy, and peripheral nerve injury. Such pain has been attributed to increased activity in mechanoceptors and nociceptors within the neural sheath.
Pallor, cyanosis, hypo-aesthesia and abnormal sweating are commonly seen in the skin overlying a source of deep somatic pain. For example, these phenomena occur commonly and normally in a limb immobilized after a fracture (the immobility being an important causal feature), and they are invariably present in complex regional pain syndromes. When present in fibromyalgia syndrome, these signs may be explained as somatic referred pain.
There are several related conditions, which have been referred to as chronic overlapping pain conditions or functional pain disorders. Their commonly associated symptoms include fatigue, sleep disorder, temporomandibular joint dysfunction, post-exertion malaise and muscle pain, numbness and tingling, skin sensitivities, morning stiffness, irritable bowel, chronic headaches (tension type or migraine), cognitive or memory impairment, menstrual cramping and premenstrual syndrome, dizziness or impaired coordination (Fig. 1).

Fig. 1. Overlapping symptoms commonly associated with fibromyalgia.
Fibromyalgia syndrome affects 2%-6% of the U.S. population depending on the diagnostic criteria: 3.4% of women; 0.5% of men, and 1.2% of children. Fibromyalgia has considerable costs, estimated at more than $16 billion annually. Treatment is very expensive, and the condition has adverse effects on employment.
5 Affected patients had 30% shorter work hours and did less demanding work; 15% received some forms of disability payment.
As noted above, many conditions can cause widespread pain, such as cervical facet joint arthropathy following whiplash, myofascial pain syndrome, myopathy from statin drugs, opioid induced hyperalgesia, hypothyroidism, parasitic infections, hepatitis, and other rheumatic diseases (Fig. 2).

Fig. 2. Differetial diagnosis of fibromyalgia.
Therefore, further laboratories studies may be needed, such as CBC, TSH, LFT, ESR to rule out inflammatory arthritis; and vitamin D, vitamin B12, and Fe level to rule out chronic anemia.
PATHOPHYSIOLOGY
Fibromyalgia is a heterogeneous condition which likely has multiple potential etiologies. Many clinical studies suggest that various individuals with chronic pain, including fibromyalgia, will have a mix of both peripheral nociceptive augmentation and centrally driven augmentation.
The evidence of muscle dysfunction in fibromyalgia is based on biopsies, with findings of decreased high energy phosphates and elevated substance P.
6
There is also strong evidence
7 that the central nervous system is a major component of the pain. Fibromyalgia is associated with altered processing in the CNS, with enhanced excitability and decreased inhibition, manifest as hyperalgesia and central sensitization. The CSF in these patients shows elevation of substance P and nerve growth factor, and decreased serotonin.
8 Central sensitization is claimed to involve ‘wind-up phenomena’ due to activation of N-methyl-D-aspartate receptors on second-order neurons in the brainstem (Fig. 3).
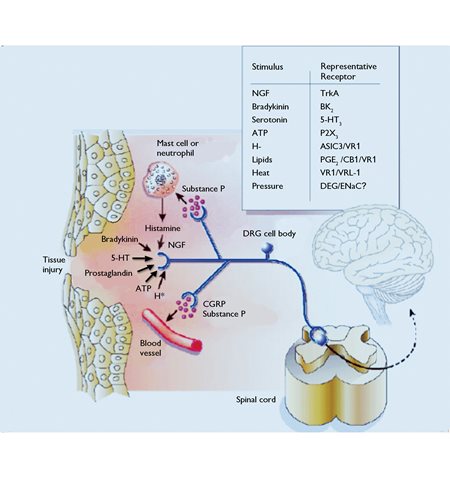
Fig. 3. The neurosecretory pathway of sensory afferents from tissue injury.
Studies have found abnormal levels of cytokines, changes in DNA methylation, activation of the hypothalamic-pituitary-adrenal system, differences in neuropeptides, subcortical neuronal changes, and genetic-environmental interactions to be involved in the pathogenesis of fibromyalgia. Several studies found elevated plasma levels of cytokines, which points to chronic systemic inflammation.
9 Supporting evidence indicates there is cortical or subcortical augmentation of pain processing. Dysfunction of descending pain-modulating pathways due to central serotonin deficiency could lead to chronic deep pain.
Importantly, the underlying cause of these variations is still unknown. There seem to be increased rates of fibromyalgia in family members of patients, and several genes have also been implicated as potentially involved in its pathogenesis.
It is important to understand both the peripheral and central contributions in individuals with chronic pain, so that therapies can be targeted toward both peripheral and central components, as well as any accompanying co-morbid conditions such as anxiety.
THE ROLE OF STRESS AND DEPRESSION
Stress
Fibromyalgia has often been described as a stress-related disorder, and altered stress systems have been viewed as causing pain and other symptoms experienced in this condition. Many patients who present with fibromyalgia symptoms and have normal laboratory and neuroimaging studies have heard at some point that their symptoms are “all in their heads” or that their symptoms are from stress or depression.
The body’s two stress systems, the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nervous system, are indeed altered in fibromyalgia.
10 For both systems, hyper and hypo activity in basal functioning and acute stress responses have been reported. Concerning the HPA axis, it has been suggested that prolonged periods of stress are associated with heightened basal tone and exaggerated acute stress responses.
Social and environmental variables such as a history of early trauma or neglect, or chronic physical or emotional stress as an adult, are postulated to be involved in the development of fibromyalgia.
11,12,13 Chronic stress and adverse childhood experiences (ACE) can affect the hypothalamic-pituitary-adrenal axis which may impact nociception and pain processing. Although the occurrence of ACEs is high among individuals with fibromyalgia, the development of PTSD has a greater relationship to self-reported pain severity.
12
Depression
It is not surprising that commonalities have been identified between depression and fibromyalgia, despite some clear distinctions. For instance, serotonin is involved with regulation of sleep, mood, and pain perception, and, as noted, individuals with fibromyalgia have increased levels of substance P, which is released during stimulation of 5-HT3 receptors.
14
In addition, compared with fibromyalgia alone, concurrent fibromyalgia and depression is associated with greater symptom severity, lower quality of life, and poorer fitness levels.
15 Although depression is commonly diagnosed in those with fibromyalgia, some individuals with fibromyalgia may never have depression and vice versa. Though depression and fibromyalgia are both associated with sleep disturbances, individuals with fibromyalgia have poorer quality of sleep than those with depression alone.
16
Nonetheless, there is a strong relationship between depression and fibromyalgia. Perhaps unsurprisingly, Adler-Neal et al (2019)
17 found that individuals with greater depressive symptoms reported higher levels of pain sensitivity and unpleasantness to experimental pain. These results were associated with greater activation in the ventrolateral prefrontal cortex, anterior insula, secondary somatosensory cortex, and posterior insula, suggesting both executive and sensory processes may impact pain and depressive symptoms.
In sum, a combination of genetic and environmental factors contributes to the onset of fibromyalgia and progression of symptoms. Psychological trauma can be a triggering event for the onset of symptoms and the neurochemical and hormonal effects of chronic stress may also impact the severity of symptoms.
FIBRO FOG
The term “fibro fog” refers to subjective cognitive changes in attention, memory, and word-finding ability experienced by patients with fibromyalgia. In various studies, fibromyalgia has been associated with greater impairments in executive functioning, sustained attention, memory, mental processing speed, and language skills.
18
One theory suggested that psychiatric comorbidity might explain the discrepancies, but patients with fibromyalgia had greater dysfunction on tests of cognitive flexibility and inhibition than patients with depression.16 Pain severity may also be a mediator for attentional abilities and therefore may account for some degree of the reported cognitive dysfunction.
Another study suggested that BMI and pain severity have a greater impact on test performance than sleep disturbance, fatigue, depression, or anxiety.
18 Another recent preliminary study suggested that insulin resistance may be involved in the development of symptoms.
19
It is possible that different genotypes and phenotypes of fibromyalgia exist, which might better explain the wide range of symptoms and severity seen in practice and research.
In addition to the varied findings of cognitive dysfunction, there are different theories as to the mechanism by which cognitive dysfunction may occur. Studies have found significant gray matter loss in the brains of individuals with fibromyalgia, which increases with the duration of the disease.
20 Neuroimaging studies reveal that – compared with healthy control subjects – fibromyalgia patients have reduced gray matter density in the cingulate cortex, insular cortex, medial frontal cortex, and the parahippocampal gyri;
20 and prolonged activation on fMRI in the insular-basal ganglia region and cingulate cortex to pressure stimulus.
21 There may also be changes in cerebral perfusion. Transcranial Doppler sonography found differences in cerebral blood flow volumes in individuals with fibromyalgia compared with controls.
22
Overall, though patients commonly report problems with mental alertness, attention, and memory, the research is unclear regarding how structural, chemical, and circulatory processes may contribute to these cognitive complaints.

New Clinical Fibromyalgia Diagnostic Criteria Part 1, & Symptom Severity Score Part 2a.
 Click here to Print Adobe PDF
Click here to Print Adobe PDF

New Clinical Fibromyalgia Diagnostic Criteria Part 2b Symptom Severity Score.
 Click here to Print Adobe PDF
CONCLUSION
Click here to Print Adobe PDF
CONCLUSION
Soft tissue pain as a consequence of sprain or other trauma is usually short-lived. Persistent or recurrent soft tissue pain is undoubtedly a genuine clinical entity. When it is poorly localized, chronic, deep, and aching, and lacks both internal and external objective validation, it is commonly ascribed to fibromyalgia or myofascial pain syndrome.
The pathophysiology of this condition seems to involve both peripheral (nerve, muscle) and central (cortical, hypothalamic-pituitary-adrenal) components that are still incompletely defined. In addition, stress and depression undoubtedly play a role in causation, though their relative importance is not yet fully elucidated. Finally, there is likely an aspect of the disease that involves structural changes in the CNS, and as yet ill-defined effects on mentation.
In a subsequent article, we will discuss further aspects of pathophysiology and explore the current approaches to therapy.
REFERENCES
1. Gowers WR: Lumbago: Its lessons and analogues.
Br Med J. 1904; 1:117–121.
2. Bach F: The Rheumatic Diseases: Their Recognition and Treatment. London, Cassell, 1935, pp 4, 6.
3. Copeman WSC. Aetiology of the fibrositic nodule: A clinical contribution.
Br Med J. 1943; 2: 263–264
4. Wolfe F, Smythe H, Yunus MB, et al. The American College of Rheumatology 1990 criteria for the classification
of fibromyalgia. Report of the multicentre criteria committee.
Arthritis Rheum 1990; 33:160–172.
5. Wolfe F, Anderson J, Harkness D, et al. A prospective, longitudinal, multicenter study of service utilization and costs in fibromyalgia.
Arthritis Rheum.1997; 40 (9):1560-1570
6. Bengtsson A. The muscle in fibromyalgia.
Rheumatol. 2002;41(7): 721-724. https://doi.org/10.1093/rheumatology/41.7.721
7. Sluka KA, Clauw DJ. Neurobiology of fibromyalgia and chronic widespread pain.
Neuroscience. 2016; 338:114–129. doi:10.1016/j.neuroscience.2016.06.006
8. Russell IJ, Orr MD, Littman B, et al. Elevated cerebrospinal fluid levels of substance P in patients with the fibromyalgia syndrome,
Arthritis Rheum. 1994. 37(11):1593–1601.
9. Ernberg M, Christidis N, et al. Plasma cytokine levels in fibromyalgia and their response to 15 weeks of progressive resistance exercise or relaxation therapy.
Mediators of Inflammation, 2018; Article ID: 3985154. 14 pages. https://doi.org/10.1155/2018/3985154
10. Williams DA, Clauw DJ. Understanding fibromyalgia: lessons from the broader pain research community.
J of Pain. 2009;10(8): 777–791. https://doi.org/10.1016/j.jpain.2009.06.001
11. Chang MH, Hsu JW, et al. Bidirectional association between depression and fibromyalgia syndrome: a nationwide longitudinal study.
J Pain. 2015; 16: 895–902.
12. Coppens E, Van Wambeke P, et al. Prevalence and impact of childhood adversities and post-traumatic stress disorder in women with fibromyalgia and chronic widespread pain.
European J Pain. 2017; 21(9): 1582–1590.
13. D’Agnelli S, Arendt-Nielsen L, et al. Fibromyalgia: Genetics and epigenetics insights may provide the basis for the development of diagnostic biomarkers.
Molecular Pain. 2019; 15: 1-12.
14. Khalid S, Simonds E, et al. The clinical anatomy of fibromyalgia.
Clin Anat. 2018; 31(3): 387–391.
15. Del Pozo-Cruz J, Alfonso-Rosa RM, et al. Depression symptoms are associated with key health outcomes in women with fibromyalgia: a cross-sectional study.
Internat J Rheum Dis. 2017; 20(7): 798–808
16. Gelonch O, Garolera M, et al. The effect of depressive symptoms on cognition in patients with fibromyalgia.
Plos One. 2018; 13(7), e0200057.
17. Adler-Neal AL, Emerson NM, et al. Brain moderators supporting the relationship between depressive mood and pain.
Pain. April 30, 2019.
18. Muñoz Ladrón de Guevara C, Fernández-Serrano MJ, et al. Executive function impairments in fibromyalgia syndrome: Relevance of clinical variables and body mass index.
Plos One. 2018; 13(4): e0196329
19. Pappolla MA, Manchikanti L, et al. Is insulin resistance the cause of fibromyalgia? A preliminary report.
Plos One. 2019; e0216079.
20. Kuchinad A, Schweinhardt P, et al. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain?
Journal of Neuroscience. 2007; 27(15): 4004-7.
21. Pujol J, Lopez-Sola M, et al. Mapping brain response to pain in fibromyalgia patients using temporal analysis of fMRI.
Plos One. 2009; 4(4): e5224.
22. Montoro CI, Duschek S, et al. Cerebral blood flow variability in fibromyalgia syndrome: Relationships with emotional, clinical and functional variables.
Plos One. 2018; 13(9): e0204267.