
Winter 2019 - Vol. 14, No. 4
Candida Auris:
A New and Emerging Fungal Pathogen
Joseph M. Kontra, M.D.
Chief, Division of Infectious Diseases
Medical Director of Infection Prevention
Medical Director of Microbiology
Penn Medicine Lancaster General Health Physicians
If it is a terrifying thought that life is at the mercy of the multiplication of these minute bodies [microbes], it is a consoling hope that Science will not always remain powerless before such enemies...
— Louis Pasteur
INTRODUCTION
Candida auris is a newly recognized, novel fungal pathogen that has proved capable of causing protracted and tenacious nosocomial outbreaks with high associated mortality. This paper describes the origins and spread of this pathogen, and the unique features that have made it a burgeoning global health threat.
ORIGINS AND GLOBAL SPREAD
The first isolation of this new species,
Candida auris, is attributed to a 2009 report of an ear canal culture from a woman in Japan.
1 However, a retrospective analysis of Candida isolates from South Korea has identified cases dating back to 1996, including the case of an invasive bloodstream infection in a Korean child.
2
The evolutionary spark for the origin and global spread of
C. auris remains enigmatic. Rather than originating from a single point of origin, four unique clades of
C. auris simultaneously appeared in geographically distinct regions on three continents around the globe. Clades appeared in East Asia, India, South Asia, and in South Africa. Interestingly, genomic analysis has demonstrated wide variation (thousands of single nucleotide polymorphisms) between individual clades.
3 Genetic variation within clades, however, is minimal, consistent with their emergence as four independent evolutionary events.
Many origin theories have been proposed. These include the selection pressure of widespread agricultural antifungal use, selection of thermo-tolerant strains by the rising temperatures of global warming, and transplantation of thermo-tolerant strains by migrating birds.
4 While there is no proof of these or any other origin theories, it is worth noting that
C. auris replicates best at 42°C, rather than the 37°C preferred by other Candida species.

Fig. 1. Countries from which Candida auris cases have been reported, as of August 31, 2019. Source: The Centers for Disease Control and Prevention.
The rapid global spread of this novel yeast has been astonishing. From 2009 to 2015, C. auris spread from a few initial foci to five continents (Fig. 1). Cases in the United States first appeared in 2016, predominately in the New York City, Chicago, and New Jersey regions.
5 At this time (fall 2019), cases of invasive C. auris have been documented in 12 states, including over 750 confirmed cases and over 1,500 colonized patients, as tallied by the CDC. To date there have been no reported cases in Pennsylvania (Fig. 2).

Fig. 2. Clinical cases of Candida auris reported by U.S. states as of July 31, 2019. Source: The Centers for Disease Control and Prevention.
UNIQUE FEATURES
The emergence and rapid spread of C. auris is extraordinary for a fungal pathogen. Several distinctive and disquieting characteristics of this new yeast that have emerged from recent research have allowed us to begin to unravel the puzzle of its ascendency as a lethal pathogen. These are summarized in Table 1, and will be addressed subsequently.
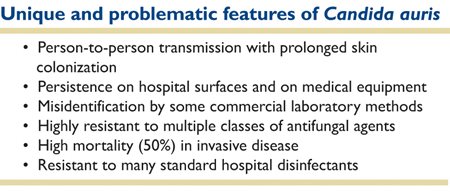
Table 1. Distinctive and disquieting characteristics of this new yeast.
MICROBIOLOGY
The genus Candida consists of over 500 species, although only about half a dozen commonly cause disease in humans. Colonies of
C. auris are indistinguishable from other common Candida species, and it does not form pseudo-hyphae or germ tubes.
C. auris is commonly misidentified by commercial biochemical (phenotypic) identification systems, most commonly as
Candida haemulonii, to which it is closely related phylogenetically.
The incorrect species designation varies among the different FDA-approved commercial identification systems, and at least 11 common yeast species have been described as false results. Fortunately, identification by Matrix-Assisted Laser Desorption Ionization-Time of Flight (MALDI-TOF) mass spectroscopy has now been FDA-approved as an accurate diagnostic method. Molecular methods based on 28S ribosomal sequencing are also being developed, and presumptive identification of
C. auris directly from smear-positive blood cultures is now available.
6 The laboratories of the CDC can also be utilized for guidance and validation. In the Lancaster General Hospital Microbiology Lab, both MALDI-TOF and direct blood PCR (polymerase chain reaction) are available to optimize the rapid diagnosis of
C. auris.
VIRULENCE FACTORS
Growth of
C. auris occurs in one of two morphologic patterns, aggregative and non-aggregative. In the former, daughter yeast cells are not released after budding, but rather form dense clusters that are difficult to disrupt
in vitro. The non-aggregative growth pattern, however, has been found to be more capable of forming a biofilm, and in animal models demonstrates far greater pathogenicity.
7 In vivo development of a more invasive filamentous morphology has also been described.
C. auris can produce a phospholipase that enhances its adhesiveness, and its ability to invade host cells.
8 Finally,
C. auris is much more effective than other Candida species at evading neutrophil phagocytosis.
9 Further research will undoubtedly reveal additional mechanisms of virulence.
CLINICAL SIGNIFICANCE
Candida species have always been a significant cause of nosocomial bloodstream infections,
10 but in the past these were generally the result of overgrowth and opportunistic invasion by commensal Candida in debilitated, critically ill patients. Human-to-human transmission had not been previously considered important epidemiologically. A crucial distinction about
C. auris infections is that they are exogenous, whereas most other Candida infections result from endogenous flora.
In only a few years,
Candida auris has gone from being a pathogen no one heard of to one that causes up to 40% of invasive Candida infections in some international centers. The role of biofilms in pathogenic strains is highlighted by the clear association between invasive
C. auris infections and intensive care settings, especially in patients with central venous catheters or indwelling Foley catheters. But these clinical risk factors are similar to other Candida species, and do not allow for differentiation at the bedside. Rather, epidemiologic clues are crucial in establishing a high index of suspicion for
C. auris infection.
Risk factors for colonization and disease include a history of hospitalization in a country or region known to harbor
C. auris (Fig. 1). While many countries have now reported cases, C. auris infections in the United States have been identified in patients with recent health care exposures specifically in India, Pakistan, Kenya, Kuwait, South Africa, the United Arab Emirates, and Venezuela.
11
While mortality rates vary by geographic region, combined reports from the Far East, Asia, and the United States suggest mortality rates of approximately 50% for invasive
C. auris infections. Sites of infection have included primary or catheter-associated bacteremias, the urinary tract, abdomen, and wounds.
12 Colonization with
C. auris portends a high risk of subsequent infection, which occurs in about half of colonized patients.
ANTIFUNGAL SUSCEPTIBILITY AND TREATMENT OPTIONS
High-level multi-drug resistance is another defining feature of
C. auris. This organism has demonstrated, to varying degrees, clinical resistance to all three classes of antifungals, although isolates vary regionally. All isolates should be subjected to antifungal susceptibility testing. Unfortunately, however, there are no
C. auris-specific breakpoints yet established by the Clinical Laboratory Standards Institute (CLSI); there are still insufficient data about the correlation between Minimum Inhibitory Concentration (MIC) and clinical outcomes. In the meantime, based on data from other Candida species, tentative MIC breakpoints have been established.
In U.S. isolates thus far, about 90% of C. auris are resistant to fluconazole, and about 30% have been resistant to amphotericin B. Resistance to echinocandins is much less common at 5%. Development of pan-resistance during treatment is a well-described phenomenon in at least 10% of cases.
13 Because of the latter scenario, in vitro investigations into possible combination antifungal therapy are being performed. The combination of micafungin and voriconazole has shown promise in laboratory testing.
14
The last iteration of clinical practice guidelines on the management of candidiasis published by the Infectious Diseases Society of America
15 did not provide guidance on the management of
C. auris, and updated recommendations are needed. In the interim, treatment strategies have emerged based on accumulating clinical experience.
16 An echinocandin antifungal is appropriate first line therapy, with the most experience reported with micafungin. Pharmacodynamic considerations, however, caution against using micafungin for central nervous system or urinary infections due to poor penetration into these sites. For central nervous system infections, liposomal formulations of amphoterecin B with flucytosine are preferred. Posaconazole or isavuconazole could be considered alternative agents if supported by susceptibility data.
A new 1,3-beta-D-glucan synthesis inhibitor, Ibrexafungerp (formerly SCY-078), has excellent
in vitro activity against all clades of
C. auris, and is highly bioavailable with enteral dosing.
17 Other potential antifungals in the pipeline include fosmanogepix (APX001), which inhibits fungal cell membrane synthesis
18, and MYC-053, which has broad antifungal activity and has been shown to inhibit fungal biofilms.
19
EPIDEMIOLOGY AND INFECTION CONTROL
Efficient human-to-human transmission of
C. auris is yet another defining feature of this new pathogen, and one that is the cornerstone of its ability to cause nosocomial outbreaks of invasive disease.
C. auris can colonize any site in the body, and can persist for more than three months even after systemic fungicidal treatment. Invasive infections have been documented within as little as 48 hours from admission to an ICU where
C. auris transmission is present.
20 This pathogen can survive on dried hospital surfaces for up to two weeks.
C. auris has been persistently recovered from hospital floors, walls, furniture, mattresses, and reusable medical equipment. As an example, an outbreak of invasive
C. auris infection and persistent nosocomial colonization of patients in a neuroscience ICU in the United Kingdom was traced to reusable skin surface axillary temperature probes.
21 The epidemic was finally halted by discarding the contaminated probes.
Viability testing of
C. auris has demonstrated a fascinating ability of yeast cells to enter a metabolically active but non-cultivatable state for up to four weeks.
22 To further complicate matters,
Candida auris is resistant to a wide range of standard hospital disinfectants, including alcohol and quaternary ammonium compounds, which hindered early attempts at outbreak control. Similar to the approach used for contamination of hospital environments with
Clostridioides difficile spores, terminal cleaning for
C. auris with various combinations of bleach, hydrogen peroxide vapor, and UVC radiation has proven effective.
23 Contaminated textile surfaces such as sphygmomanometer cuffs are best discarded.
CDC recommendations for infection control and prevention of
Candida auris are summarized in Table 2.
24
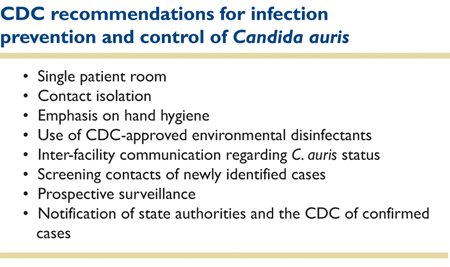
Table 2. These recommendations will evolve as more information about C. aris becomes available.
These must be considered interim recommendations, and certainly will evolve with time. Many issues remained unanswered. Transmission of
C. auris by health care workers (HCW) is poorly defined, but must certainly play a role.
25 For patients, it is not clear which body sites should be screened and how frequently surveillance cultures should be performed. Decolonization protocols remain undefined. And while the CDC has proposed surveillance cultures and attempts at decolonization every three months, conclusive data are lacking on the impact of those proposals. For these and other reasons, the duration of contact precautions for colonized patients remains undefined, although many infection control professionals would consider the contact isolation requirement to be lifelong.
Furthermore, proper management of colonized patients or HCW is unclear, and certainly will be problematic for this multidrug-resistant pathogen. Proactive surveillance cultures for patients admitted from high-risk facilities, which can include extended care facilities, will be the key to heading off an outbreak. A single confirmed isolate of
C. auris in a facility should result in initiation of patient and contact screening. Unfortunately, at present there are no commercially available, selective media capable of rapid screening of surface specimens for
C. auris. Once
C. auris is identified in an ICU, microbiology lab protocols will require modification. All yeast isolates from that ICU should then be identified to the species level in order to detect newly colonized patients. These labor-intensive responses to
C. auris are likely just the tip of the iceberg, and much research lies ahead to truly understand how to manage this pathogen. Fig. 3 summarizes one proposed management algorithm for suspected or confirmed
C. auris cases.
26

Fig. 3. Management of Suspected and Confirmed Candida auris. Source: JAMA. 2019; 322(15: 1510-1511. doi: 10. 1001/jama.2019. 13843. © 2019 American Medical Associaton.
CONCLUSIONS
Candida auris has emerged rapidly as an increasingly important cause of morbidity and mortality worldwide, especially in intensive care settings. Unique virulence factors, tenacious persistence in the hospital environment, and resistance to multiple classes of antifungal agents, have elevated
C. auris to a high threat level of concern. This pathogen is, and will likely remain, a challenge for microbiologists, infectious disease practitioners, intensivists, public health authorities, and infection control professionals.
REFERENCES
1. Satoh, K, Makimura K, Hasumi Y, et al.
Candida auris sp. nova, a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital.
Microbiol Immunol. 2009; 53:41-44.
2. Lee WG, Shin JH, Uh Y, et al. First three reported cases of nosocomial fungemia caused by Candida auris.
J Clin Microbiol 2011;49: 3139-3142.
3. Lockhart SR, Etienne KA, Vallabhaneni S, et al. Simultaneous emergence of multidrug-resistant
Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses.
Clin Infect Dis. 2016; 64:134-140.
4. Cassadevall A, Kontoyiannis DP, Robert V, et al. On the emergence of
Candida auris: climate change, azoles, swamps, and birds.
mBio.2019;10(4): e01397-19
5. Centers for Disease Control and Prevention.
Candida auris. https://www.cdc.gov/fungal/candida-auris/tracking-c-auris.html#world
6. Centers for Disease Control and Prevention.
Candida auris. https://www.cdc.gov/fungal/candida-auris/recommendations.html
7. Nett JE.
Candida auris: an emerging pathogen ‘incognito’?
Plos Pathog.2019;15e1007638.
8. Rosato L, Colombo AL.
Candida auris: what have we learned about its mechanisms of pathogenicity?
Front Microbiol 2018;9: 3081
9. Johnson CJ, Davis JM, Huttenlocher A, el al. Emerging fungal pathogen
Candida auris evades neutrophil attack.
mBio 2018;9: 1-9.
10. Magill SS, Edwards JR, Bamberg W, et al; Emerging infections program healthcare-associated infections and antimicrobial use prevalence survey team. Multistate point prevalence survey of healthcare-associated infections.
N Engl J Med. 2014; 370:1198-1208.
11. Centers for Disease Control and Prevention.
Candida auris. https://www.cdc.gov/fungal/candida-auris/tracking-c-auris.html
12. Vallabhaneni S., Kallen A, Tsay S, et al. Investigation of the first seven reported cases of
Candida auris, a globally emerging invasive, multidrug-resistant fungus – U.S. May 2013-August 2016. MMWR 65:1234-37.
13. Centers for Disease Control and Prevention.
Candida auris.https://www.cdc.gov/fungal/candida-auris/c-auris-antifungal.html
14. Fakhim H, Chowdhary A, Prakash A, el al. In vitro interactions of echinocandins with triazoles against multidrug-resistant Candida auris. Antimicrob Agents Chemother 2017. Doi:10.1128.AAC.01056-17.
15. Pappas PD, Kauffman CA, Andes DR, et al. Clinical practice guidelines for the management of candidiasis: 2016 update by the infectious diseases society of America.
Clin Infect Dis 2016. 62(4):e1-e50.
16. Corsi-Vasquez G, Ostrosky-Zeichner L.
Candida auris: what have we learned so far?
Current Opinion Infect Dis. 2019;32:1-6.
17. Larkin E, Hager C, Chandra J, et al. The emerging pathogen
Candida auris: growth phenotype, virulence factors, activity of antifungals, and effect of SCY-078, a novel glucan synthesis inhibitor, on growth morphology and biofilm formation.
Antimicrob Agents Chemother 2017;61: e02396-17.
18. Hager CL, Larkin EL, Long L, et al. In vitro and in vivo evaluation of the antifungal activity of APX001A/APX001 against
Candida auris. Antimicrob Agents Chemother 2018;62: 1-7.
19. Tetz G, Collins M, Vikina D, et al. In vitro activity of a novel antifungal compound, MYC-053, against clinically significant antifungal resistant strains of Candida glabrata,
Candida auris, Cryptococcus neoformans, and Pneumocystis spp.
Antimicrob Agents Chemother 2019;63:1-8.
20. Jeffery-Smith A, Taori SK, Schelenz S, et al. Candida auris: a review of the literature.
Clin Microbiol Rev 2017;31: 1-17.
21. Eyre DW, Sheppard AE, Madder H, et al. A
Candida auris outbreak and its control in an intensive care setting.
N Engl J Med 2018;379: 1322-1331.
22. Ruiz-Gaitan A, Martinez H, Moret AM, et al. Detection and treatment of
Candida auris in an outbreak situation: risk factors for developing colonization and candidemia by this new species in critically ill patients.
Expert Rev Anti Infect Ther 2019;17: 295-305.
23. Spivak ES, Hanson KE. Candida auris: an emerging fungal pathogen.
J Clin Microbiol 2018;56: 1-10.
24. Centers for Disease Control and Prevention.
Candida auris.
https://www.cdc.gov/fungal/candida-auris/c-auris-infection-control.html
25. Jeffery-Smith A, Taori SK, Schelenz S, et al;
Candida auris Incident Management Team.
Candida auris: a review of the literature.
Clin Microbiol Rev. 2017;31(1): e00029-e17.
26. Bradley SF. What is known about Candida auris.
JAMA Insights. Clinical Update. 2019.
https://jamanetwork.com/journals/jama/fullarticle/2749798