Summer 2019 - Vol. 14, No. 2


Dermody Wood
Transcarotid Artery Revascularization (TCAR)
An Endovascular Alternative to Carotid Endarterectomy for High-Risk Patients
Meghan Dermody, MD, MS, RPVI, FACS
Chief, Division of Vascular Surgery
Co-Medical Director, Intervention Vascular Unit
Penn Medicine LG Health Physicians Surgical Group
Todd Wood, MD, FACC, FSCAI, FSVM, RPVI
Chief, Division of Cardiology
The Heart Group of Lancaster General Health
Heart and Vascular Institute, Lancaster General Health
INTRODUCTION
Carotid artery atherosclerosis that causes stenosis or occlusion creates a higher risk for recurrent cerebral ischemia than any other subtype of stroke. Population-based studies estimate that approximately 15% of ischemic strokes are due to large vessel (i.e. internal carotid artery) disease, with two-thirds of those due to stenosis, and one-third due to occlusion. Conservative estimates attribute approximately 41,000 strokes annually in the United States to extracranial internal carotid artery stenosis.
1
Multiple randomized-controlled trials have confirmed that carotid endarterectomy (CEA) decreases the risk of recurrent stroke in patients with symptomatic internal carotid artery stenosis, ideally when it is performed within the first two weeks following the event. However, only 15% of stroke victims have a transient ischemic attack (TIA) prior to a permanent stroke. A study published in the April 2013 issue of
Stroke showed the overall stroke risk in patients with extracranial internal carotid artery stenosis to be 2.2% over an observation period of 6.3 years. For those patients with ≥70% stenosis, the
annual stroke rate was 0.5%. Half of all strokes in this population were due to large vessel disease.
2
In the current era of maximal medical management with cholesterol-lowering agents and antiplatelet medications, symptomatic events are becoming less common, and indications for interventions in asymptomatic patients with carotid stenosis are decreasing. There are on-going clinical trials that compare different types of surgical management with continued medical management for asymptomatic patients, but we will not have their results for several more years. In the meantime, we continue to practice by the dictums of the multi-specialty society guidelines for carotid intervention. (Table 1)
3
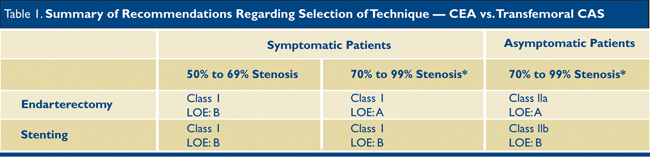
*Severity of stenosis is defined according to angiograpic criteria used in the NASCET trial but generally corresponds to assessment by sonography.
CAROTID ENDARTERECTOMY PITFALLS
For decades, the gold standard for the treatment of carotid stenosis has been CEA. This procedure has well-established complication rates that stem primarily from dissection of the carotid bifurcation around several nerves, as well as the need to sew a long patch onto the endarterectomized bifurcation. Recently reported cranial nerve injury rates range from 2.7% to 5.3%.
4,5,6 The most common nerve injuries cause hoarseness, tongue deviation, and mouth drooping. Most are due to retraction on the affected nerve, and are therefore temporary, with persistent neuropathy beyond six months occurring in 1.9%-2.1% of patients.
4,5 Bleeding is also a major complication, with the potential for airway compromise.
More important, however, is the inherent stroke risk with endarterectomy. One of the most commonly cited randomized-controlled trials evaluating CEA risks was the CREST trial, published in 2010, which compared CEA with transfemoral carotid artery stenting (TF-CAS). The 30-day periprocedural stroke rate with CEA in standard surgical risk patients (n=371) was 2.4%.
4 In the 43,114 CEA cases entered in the Vascular Quality Initiative (a national database for vascular surgical procedures), the reported stroke rate is 1.2%. In the Society for Vascular Surgery (SVS) registry of high surgical risk patients undergoing CEA, the stroke rate is 3.6% at 30 days.
7
It is important to distinguish between standard and high surgical risk patients, because the latter have much higher complication rates in all studies. The typical high-risk patient meets one or more of the following criteria:
• Prior ipsilateral neck dissection
• Prior ipsilateral CEA
• Prior neck radiation
• Surgically inaccessible lesion
• Permanent contralateral cranial nerve injury
• Severe COPD
• Un-revascularized CAD
As a result, vascular surgeons have been searching for an alternative to CEA, which led to the development of TF-CAS. This approach was initially adopted for standard risk patients, but has since been restricted only to high surgical risk patients because of its inherently higher stroke rate in all studies comparing CEA to TF-CAS. The higher rate is due to the need to pass catheters through a diseased aortic arch, and often also through the carotid lesion itself, prior to placing a distal embolic protection filter. Because of its limitations, TF-CAS gave rise to TransCarotid Artery Revascularization (TCAR), which offers a lower risk method of revascularization.
TCAR – WHAT IS IT AND WHY IS IT IMPORTANT?
The TCAR procedure is a hybrid surgical and endovascular intervention currently approved by the Centers for Medicare and Medicaid (CMS) for patients who meet specific criteria that qualify them as high risk for surgical CEA.
The procedure involves dissection of the proximal common carotid artery ipsilateral to the stenotic lesion with insertion of a short sheath which stays proximal to the carotid bifurcation. The carotid sheath is then connected to a sheath in the femoral vein through a shunt with a filter device. Since the femoral vein is a low-pressure vessel, blood flows in a reverse direction from the brain down the internal carotid artery, across the carotid stenosis, through the carotid sheath, into the shunt, through the filter, and back into the patient via the femoral vein sheath.
This reversal of flow is the main benefit of this procedure, as it virtually eliminates the possibility of any plaque or thrombotic debris entering the brain. While flow is reversed, the lesion is crossed with a wire and treated by angioplasty and stenting, as would typically be done from the femoral approach. This procedure is usually done with conscious sedation and local anesthesia. (Fig. 1)
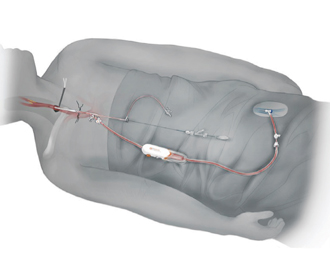
Fig. 1. Patient set up for TCAR.
To qualify for reimbursement from CMS or commercial insurers, certain clinical or anatomic criteria must be met that categorize a patient as high risk for CEA. (Table 2) Reimbursement is also linked to a post-market quality initiative, whereby centers performing these procedures must enter the patients and procedural data into the aforementioned SVS Vascular Quality Initiative database as part of the TCAR Surveillance Project.
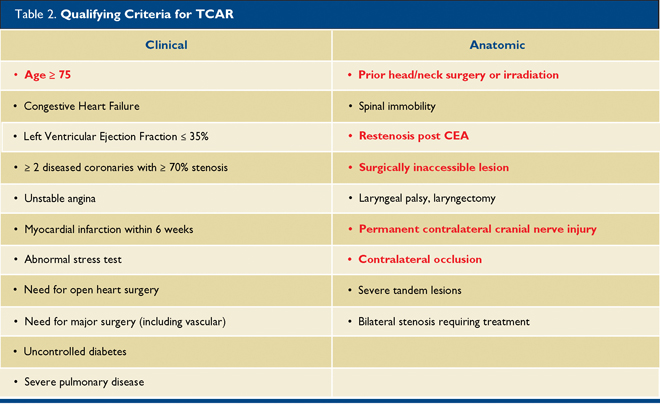
Any one risk factor qualifies a patient for CMS high surgical risk status.
CMS covers TCAR under the listing of National Coverage Determinations (NCD) – Section 20.7 – for patients in any institution who meet criteria for high surgical risk, are symptomatic, and have ≥70% stenosis. However, because Lancaster General Hospital is an approved site for the TCAR Surveillance Project, we are also able to offer TCAR to patients with high surgical risk who have either:
a) a stenosis ≥50% with symptoms, or
b) a stenosis ≥80% even without symptoms.
We can thus offer this lower risk procedure without any negative financial impact.
The TCAR procedure (DRG 035) has a reimbursement that is $4,000 higher than for CEA (DRG 038). As a result, despite TCAR’s higher equipment costs, a hospital’s margin for TCAR is approximately $250 higher than for CEA. These favorable margins do not include the added TCAR benefits of shorter OR time and shorter length of stay (reported average 1.8 days vs. 3 days for CEA), and the potential reduction in non-reimbursed complications.
RESULTS WITH TCAR
To date, five publications or presentations have reported TCAR data, but there are no randomized-controlled trials comparing CEA with TCAR. Of the available data, it is very clear that the stroke rate with TCAR is no worse (likely better) compared with CEA. The first trial that led to approval by CMS for TCAR is the ROADSTER trial published in 2015.
8 This was a prospective, single-arm, multicenter clinical trial that followed 219 high surgical risk patients undergoing TCAR for either asymptomatic carotid stenoses ≥70%, or symptomatic stenoses ≥50%. The primary end point was the composite of all stroke, myocardial infarction (MI), and death at 30 days. Secondary end points included cranial nerve injury; 30-day stroke, death, stroke/death, and MI; acute device, technical, and procedural success; and access site complications.
The overall stroke rate in the ROADSTER trial was 1.4%, the lowest reported stroke rate to date for any prospective, multicenter clinical trial of carotid stenting. Data from this trial are compared to CEA data from the aforementioned CREST trial in Table 3.
* Hierarchical i NEJM 2010;363:11-23
ii Stroke 2011;42(12):3484-90 iii Circulation 2012;125;125:2256-64
The TCAR Surveillance Project is currently ongoing, and data from the first 2,545 patients in the registry were presented at the VEITH Symposium in New York City in November 2018. Patient characteristics and unadjusted outcomes for TCAR and CEA patients in the VQI registry are displayed in Table 4.
6 Overall, patients receiving TCAR were sicker, more likely to be symptomatic, and had higher degrees of stenosis. Despite this, the stroke and death rates were the same as CEA. Patients with TCAR had a statistically significantly lower rate of cranial nerve injury, shorter procedure time, and shorter length of stay. Hemodynamically, TCAR patients had more episodes of hypotension than CEA patients; the latter had more episodes of hypertension, often leading to bleeding complications post-operatively.
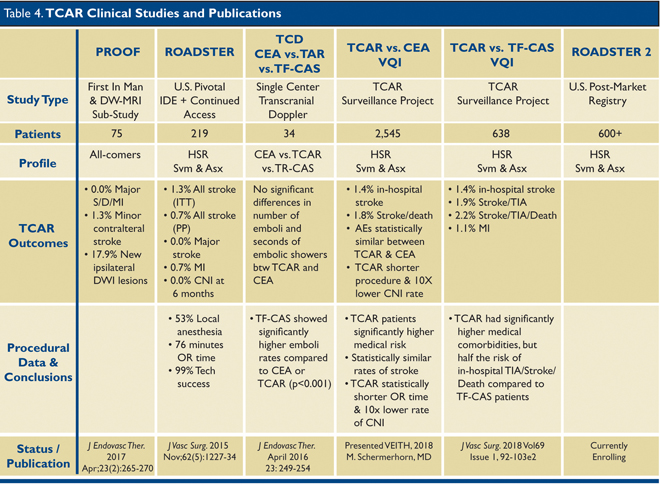
Data from the first post-approval safety and efficacy study performed at two institutions (University of Rochester and Stony Brook) were presented at the SVS Vascular Annual Meeting in Boston in June 2018,
9 and highlight some important points regarding TCAR. First, TCAR was used to treat older patients; 45% were ≥75 years old and 27% were ≥80 years old. Second, despite having a higher ratio of symptomatic patients and, on average, longer lesions to stent, TCAR still had a lower rate of stroke than CEA or TF-CAS. There was one post-operative watershed infarct reported in this study, providing a stroke rate of 1.1%. There were no deaths or MI in this study. Lastly, 32% of patients were done under local anesthesia which is typically not well tolerated for CEA.
In a First in Man experience, the PROOF study performed diffusion-weighted magnetic resonance imaging (DW-MRI) before and after TCAR in 56 patients. Of these, 10 (17.9%) had ipsilateral new white matter lesions, with a mean volume of 0.17 mL, but they did not lead to a neurological deficit. At 30 days, there were no major strokes, MI, or deaths in this cohort.
This outcome compares favorably with either TF-CAS with a proximal occlusion balloon for embolic protection, which has a 45% rate of new DW-MRI lesions post-procedure,
10 and with TF-CAS with a distal filter for embolic protection, which has a 73%-87% rate of new lesions on DW-MRI post-procedure.
11
In comparison with CEA, the five studies which report stroke rates for TCAR all show either comparable or lower rates of stroke at 30 days despite being comprised of higher risk patients. Fig. 2 shows a comparison of stroke rates among all available publications and presentations. These data are similar to the early experience we have had at LGH.
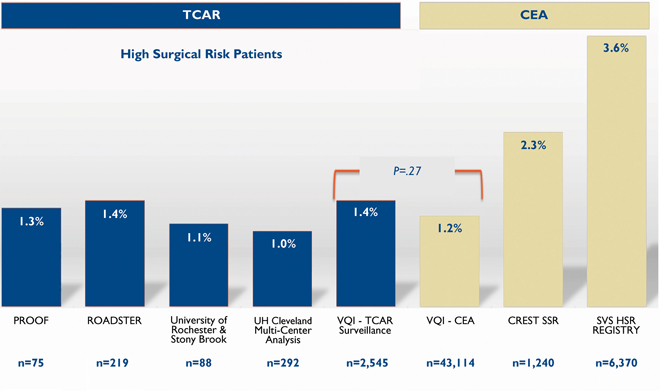
Fig.2. Periprocedural Stroke Rates of TCAR vs. CEA
PROOF:
J Endovasc Ther. 2017 Apr;24(2):265-270
VQI TCAR + CEA: In-Hospital Outcomes of TCAR & CEA in the SVS-VQI TCAR Surveillance Project – VEITH Symposium 2018 Presentation Unadjusted Outcomes – M. Schermerhorn, MD
CREST Standard Surgical Risk:
N Engl J Med. 2016 Mar 17;374(11):1011-20.
LGH EXPERIENCE
The first TCAR procedure was performed at LGH in July 2018. All cases are being entered into the VQI registry. Currently, we are on par with the regional and national benchmark data in all areas reviewed. Our procedure time is 60 minutes or less as compared with over 100 minutes for CEA. Most patients go home the following day and are able to return to work in one to two weeks (as compared with four to six weeks for CEA). All patients are loaded with clopidogrel pre-operatively and receive dual antiplatelet therapy for 30 days following stent placement. All patients are required to be on a statin drug peri-procedurally. Surveillance ultrasound is performed around three to four weeks, six months, and one year post-stenting. Most patients will have annual duplex ultrasound ordered by their surgeon indefinitely thereafter.
FUTURE MANAGEMENT OF CAROTID DISEASE
Like many other innovative minimally-invasive medical techniques, TCAR will likely be expanded to a broader population of patients in the future. With pending results from the TCAR Surveillance Project and ROADSTER-2 trial, as well as comparison with medical management in CREST-2, we hope to have more evidence-based data to support our decision-making within the next decade, with better definition of indications for intervention in asymptomatic patients.
In the meantime, options like TCAR are available at only a few regional locations. Lancaster General Hospital was credentialed as a TCAR Center of Excellence in January 2019, having performed more than 15 procedures with excellent outcomes. We will strive to continue this trend, and hope to offer this new procedure to more patients in the future.
REFERENCES
1. Flaherty ML, Kissela B, Khoury JC et al. Carotid artery stenosis as a cause of stroke.
Neuroepidemiol. 2013; 40 (1): 36-41.
https://doi.org/10.1159/000341410
2. den Hartog AG, Achtenberg S, Moll FL, et al. Asymptomatic carotid artery stenosis and the risk of ischemic stroke according to subtype in patients with clinical manifest arterial disease.
Stroke. 2013;44:1002-1007.
3. Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANS/ACR/ASNR/CNS/ASIP/SCAI/SIR/ANIS/SVM/SVS Guideline on the Management of Patients With Extracranial Carotid and Vertebral Artery Disease.
J Am Coll Cardiol 2011;57(8):e16-e94.
https://doi.org/10.1016/j.jacc.2010.11.006
4. Brott TG, Hobson RW, Howard G, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis.
N Engl J Med 2010;363:11-23. doi: 10.1056/NEJMoa0912321. Epub 2010 May 26.
5. King, AH et al. “A Multi-Institutional Analysis of TCAR Compared to CEA” VAM presentation, June 2018
6. Schermerhorn M, et al. In-Hospital Outcomes of TCAR and CEA in the SVS-VQI TCAR Surveillance Project. VEITH Symposium presentation, November 2018.
https://evtoday.com/2018/11/20/vqi-study-shows-similar-outcomes-for-tcar-versus-cea-with-lower-cni-and-or-time
7. Schermerhorn ML, Fokkema M, Goodney P, et al. The impact of Centers for Medicare and Medicaid Services high-risk criteria on outcome after carotid endarterectomy and carotid artery stenting in the SVS Vascular Registry.
J Vasc Surg 2013;57(5):1318-1324.
8. Kwolek CJ, Jaff MR, Leal JL, et al. Results of the ROADSTER multicenter trial of transcarotid stenting with dynamic flow reversal.
J Vasc Surg 2015; 62(5):1227-1234. doi: 10.1016/j.jvs.2015.04.460.
9. Balceniuk MD, et al. TCAR First Post-Approval Safety and Efficacy Study (2 Center Analysis). VAM presentation, June 2018.
10. Bijuklic K, Wandler A, Hazizi F, et al. The PROFI study (Prevention of cerebral embolization by proximal balloon occlusion compared to filter protection during carotid artery stenting): a prospective randomized trial.
J Am Coll Cardio 2012;59(15):1383-1389.
11. Bonati LH1, Jongen LM, Haller S, et al. New ischaemic brain lesions on MRI after stenting or endarterectomy for symptomatic carotid stenosis: a substudy of the International Carotid Stenting Study (ICSS).
Lancet Neurol. 2010; 9(4):353-62. doi: 10.1016/S1474-4422(10)70057-0. Epub 2010 Feb 25.
sp;