
Summer 2018 - Vol. 13, No. 2
Evaluation of the Patient with Chest Pain:
Is Conventional Stress Testing Obsolete?
Ron M. Jacob, MD, FACC, FASE, FSCCT
The Heart Group of Lancaster General Health
INTRODUCTION
Cardiovascular disease has been the most common cause of death every year since 1919.1 On average, 2,200 Americans die of CVD every day, or one every 40 seconds. Chest pain is the most common cause of ER visits, accounting for over 6 million annually, at a cost of about $10 billion.2
The diagnosis of an acute coronary syndrome is not always straightforward, and 2–8 percent of patients who present to the emergency room with an acute myocardial infarction are sent home.3 Though the gold standard for establishing the presence of coronary artery disease is invasive coronary angiography, non-obstructive disease is found in 55% of patients with chest pain who are referred for invasive angiography on the basis of an abnormal non-invasive test.4 This low yield reflects the difficulty of integrating the clinical history with the results of non-invasive testing to decide who should undergo coronary angiography, and who can be managed with medical therapy alone.
In the current environment, multimodality imaging offers multiple ways to evaluate the patient who presents with chest discomfort, including treadmill stress testing, stress echocardiography, single photon emission computed tomography (SPECT), stress perfusion magnetic resonance imaging (MRI), coronary computerized tomographic angiography (CTA), and positron emission tomography (PET). None of these methods is perfect, and each has limitations and strengths. The chosen test must be an effective gatekeeper for invasive angiography, as there is a small risk of catastrophic complications with invasive testing.5
CHOICE OF DIAGNOSTIC TESTS
Given the plethora of tests available, rather than asking “Which test should I order for my patient?” it is better to ask “What is the question I am trying to answer for this patient?”
There are three fundamental questions that a test should answer for a patient with chest discomfort:
1. Does the patient have coronary artery disease?
2. If the patient has coronary artery disease, is the lesion causing ischemia and is it therefore the cause of the patient’s chest pain?
3. Can the results of this test determine prognosis and influence management of the patient?
1. Does the patient have coronary artery disease?
A multitude of reasons other than coronary artery disease can cause chest pain, so if the first question can be answered “no,” the workup can be redirected toward non-coronary causes of chest discomfort. To answer the first question definitively, coronary CTA is better than other modalities, as 70% of patients who have an abnormal CTA will have obstructive disease on invasive angiography.4 The other tests are much less specific, and regardless of the method used, when patients are referred for angiography based on any other abnormal test, only 45% will have obstructive disease. (Table 1)
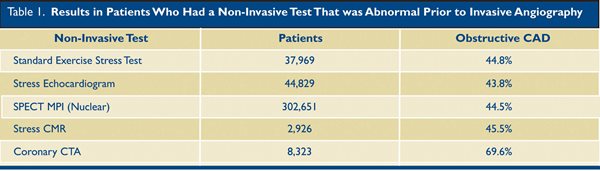
A negative Coronary CTA also has a high negative predictive value, and virtually excludes the presence of coronary artery disease as a cause of the patient’s symptoms. In contrast, a negative functional test does not exclude coronary artery disease – the patient may still have coronary artery disease that is not causing ischemia. This possibility may have ramifications in a young patient who has a high plaque burden that is non-obstructive, as the patient might be treated differently if the physician is aware of the presence of disease.
2. If the patient has coronary artery disease, is the lesion causing ischemia and is it therefore the cause of the patient’s chest pain?
A normal functional or anatomic stress test suggests non-cardiac causes for the patient’s chest discomfort. Tests that rely on myocardial perfusion imaging (MPI) to look for ischemia include SPECT (single photon computed emission tomography), PET (positron emission tomography), and CMR (cardiac magnetic resonance). Tests that rely on wall motion imaging (WMI) at peak stress include stress echocardiography and CMR (cardiac magnetic resonance). The latter, of course, also involves perfusion imaging.
Both perfusion-based tests and those that rely on wall motion abnormalities can be performed using exercise or pharmacologic stress testing. Clinical judgment should be used in interpreting a normal nuclear perfusion study, especially when the patient did not exercise, as a small subset of patients may have misleadingly balanced ischemia with multi-vessel coronary artery disease.6 Since myocardial perfusion imaging with SPECT and PET evaluates relative perfusion in myocardial segments, the study will not reveal a perfusion defect if there is reduced perfusion in all three coronary distributions, and a false negative test may result in a high risk population. It is always helpful to have the patient exercise when possible, as this process gives additional information about exercise tolerance and blood pressure response.
PREDICTIVE VALUE OF NON-INVASIVE DIAGNOSTIC TESTS
The EVINCI study compared perfusion imaging (SPECT, PET) and WMI (Stress echocardiography, CMR) and found that tests7 using perfusion imaging are more sensitive, whereas tests using WMI are more specific.
Coronary CTA has emerged as an effective way to identify coronary disease with a very high sensitivity, specificity and negative predictive value.8,9 Nonetheless, despite its high negative predictive value, CTA can have a positive predictive value for ischemia as low as 29 – 31% when patients with obstructive lesions on CTA are studied with nuclear imaging for the presence of inducible ischemia.10 (Fig. 2.)
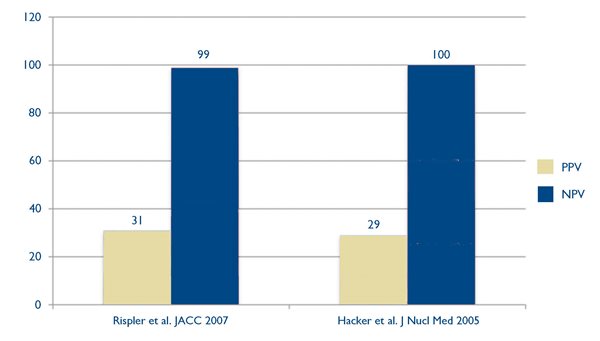
Fig. 2. Positive predictive values (PPV) in gold, and negative predictive values (NPV) in blue, in two studies 12,13 that used myocardial perfusion imaging to determine the presence of ischemia in patients with obstructive lesions identified by coronary CTA.
This poor correlation may be related to multiple factors, including image quality, coronary artery calcification, and patient-related factors.11 (Fig. 1).
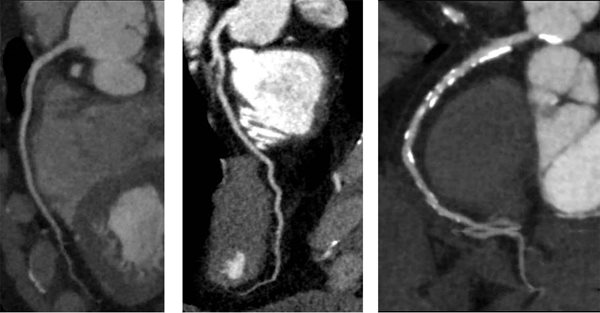
Fig. 1. Coronary CT angiograms from three patients with different disease burdens: Left panel – normal coronary arteries; middle panel – severe stenosis; right panel – a heavily calcified lesion where the degree of stenosis is difficult to determine.
The inability to identify ischemia based on the anatomic appearance of a lesion is not restricted to CT angiography. In the FAME trial, invasive fractional flow reserve measurement* found that the anatomic appearance of a moderate lesion on invasive angiography has limited ability to predict ischemia. In patients with a 50–70% stenosis, as many as 65% will have a normal fractional flow reserve (> 0.80), and will be better served by medical management.14 Several trials after the FAME trial have underscored the importance of identifying ischemia in deciding between revascularization and medical management14,15 (Table 2).
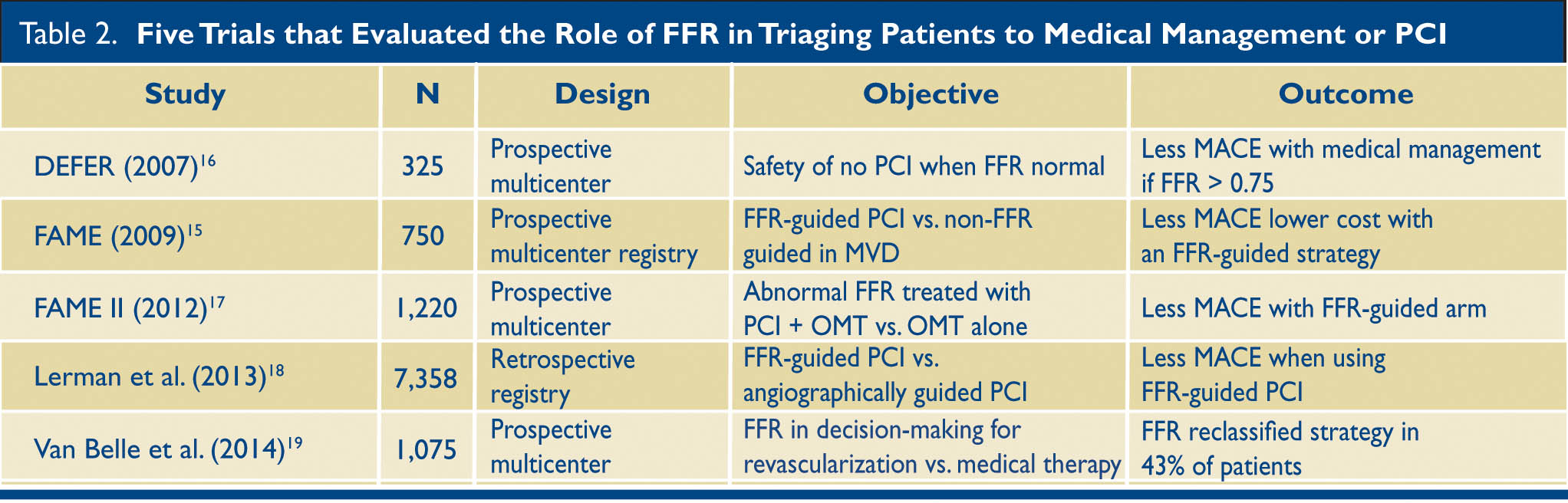
Table 2. Five Trials that evaluated the role of FFR in triaging patients to medical management or PCI. FFR = Fractional Flow Reserve; MACE = Major Adverse Cardiac Events; OMT = Optimal Medical Therapy; MVD = Multi Vessel Disease; PCI = Percutaneous Intervention
The sensitivity and specificity of some of the tests available to us are listed in Table 3.
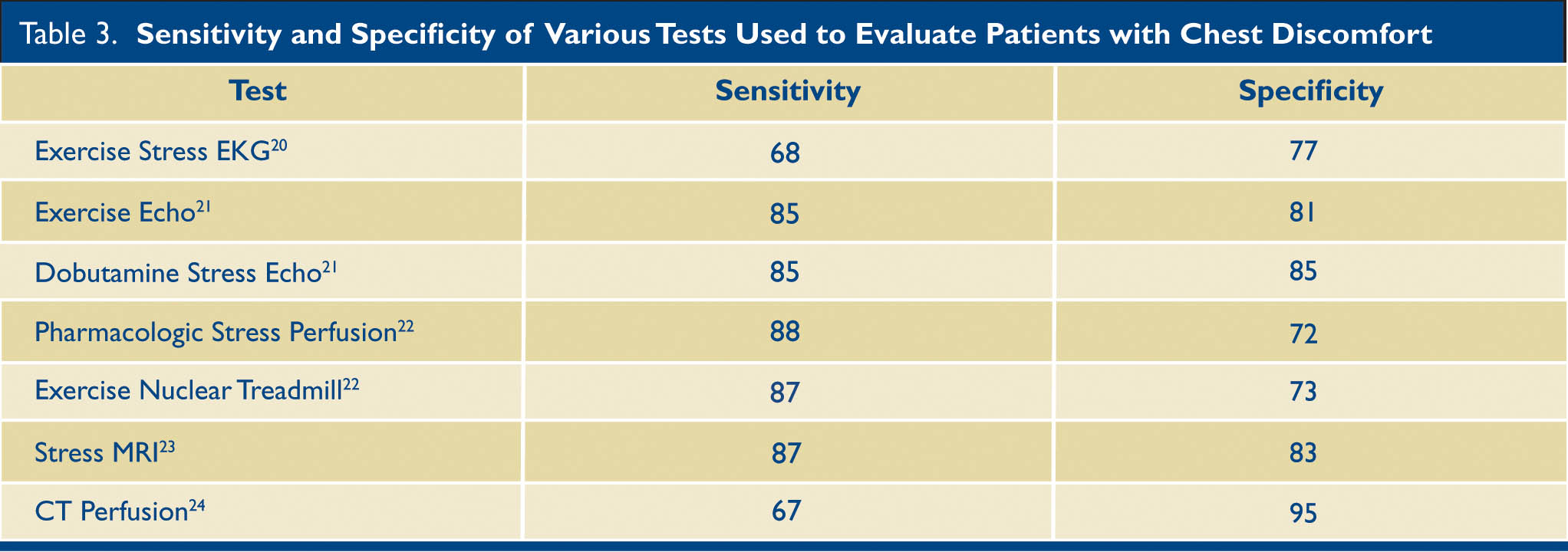
Table 3. The sensitivity and specificity of various test used to evaluate patients who present with chest discomfort. MRI —Magnetic resonance imaging; CT—Computerized tomography
NEWER CT METHODS TO DETECT ISCHEMIA: FFR–CT AND CT-PERFUSION
1. FFR–CT is based on deriving the FFR non-invasively from Coronary CT angiography by using computational fluid dynamics. This technique has been compared with invasive FFR in multiple trials, leading to FDA approval of its use to evaluate whether an obstructive lesion by Coronary CTA is causing ischemia.25 Despite being a promising technique, however, a recent meta-analysis suggested that the diagnostic agreement between FFR–CT and invasive FFR is less than ideal, and additional factors must be considered before making the decision to proceed with invasive angiography.26 Agreement between the methods is excellent for FFR values > 0.90 and < 0.60. For values between 0.90 and 0.60 there is less robust agreement between FFR-CT and invasive FFR. In this subgroup of patients, CT myocardial perfusion may be a helpful way to look for the presence of ischemia.
2. CT myocardial perfusion is a technique that is based on acquiring CT images during the first pass of iodinated contrast after the administration of a vasodilator stress agent. Areas of the myocardium that are hypo-perfused represent areas supplied by stenotic coronary arteries that are causing ischemia. (Fig. 3.)

Fig. 3. CT myocardial perfusion. In the right panel, stress images show an anterior wall perfusion defect (arrows) that is not seen on the resting images (left panel), consistent with ischemia in the LAD distribution.
Multiple studies have confirmed that the sensitivity and specificity of CT myocardial perfusion are as good or better than currently available modalities.24 More recently, investigators looked at a tiered protocol using cardiac CT and CT perfusion as an alternative to conventional functional testing.27 This approach significantly reduced the need for additional functional testing in this group of patients.
3. Can the results of this test determine prognosis and influence management of the patient?
Exercise capacity is a simple but powerful predictor of outcomes. Men with an exercise capacity below 5 METS** of exercise using a treadmill stress test have a higher mortality than men with an exercise capacity above 8 METS.28 In the CONFIRM registry, patients with non-obstructive plaques had the same rate of major cardiac events as patient’s with obstructive plaques.29 This finding suggests that patients with non-obstructive disease should be managed just as aggressively as those with obstructive disease. Even though most patients in the study had a low to intermediate pre-test probability of having coronary artery disease, 24% were found to have obstructive disease, and 26% had non-obstructive disease. This underscores the limitations of clinical assessment in patients without known coronary artery disease using the Diamond-Forrester pre-test model. When risk factor models based on clinical factors add the findings of plaque burden identified by coronary CTA, risk is reclassified in up to 49% of patients when compared with the NCEP ATP III score (National Cholesterol Education Program - Adult Treatment Panel III).30
The amount of ischemic myocardium is an important predictor of cardiovascular events, and several invasive trials have demonstrated that the presence of ischemia is an important factor in determining whether a patient should be managed medically or receive PCI (Table 2). The presence of ischemia is a continuous variable, and the severity of ischemia correlates with outcomes.31
Non-invasive studies can also be used to determine prognosis and whether an invasive strategy should be used. A seminal paper from the nuclear literature using perfusion imaging in over 10,000 patients demonstrated that patients with more than 10% of the myocardium ischemic do better with revascularization than with medical therapy. There was a direct correlation between the amount of ischemic myocardium and the survival benefit. Stress echocardiography can also be used to determine the amount of ischemic myocardium, which correlates with event free survival.32
The results of the landmark MRI–INFORM trial were recently presented. This unique randomized trial compared FFR by invasive angiography (the gold standard for determining the presence of ischemia) with stress perfusion imaging using cardiac magnetic resonance (CMR). The patients were randomized to medical management or PCI based on the presence or absence of ischemia by these two methods. The rates of death, revascularization, myocardial infarction, and MACE were the same in both arms of the study. These findings suggest that a noninvasive strategy using stress perfusion CMR is equivalent to the current invasive gold standard – FFR – in deciding which patients will benefit from PCI.
CONCLUSION
Given the vast array of choices for evaluating the patient with chest discomfort, it is important to use the right test for the right patient; no single test is appropriate for every patient. Some of the modalities described above may not be available in all centers. For a test to function as an effective gatekeeper to the cardiac catheterization laboratory, it must identify disease, correlate the disease with symptomatology, and inform the referring physician about whether medical management is better than percutaneous intervention.
For example, if the question is whether a patient with chest pain has coronary disease, if the patient has low to intermediate risk and no history of coronary artery disease, a coronary CT angiogram may be the best choice. If the test is negative, the confidence that the patient does not have coronary disease is very high, and alternative causes for the patient’s chest discomfort can we worked up. A patient with prior bypass surgery and left ventricular dysfunction who cannot exercise may be best served with pharmacologic stress perfusion testing (PET, CMR, or SPECT). Equivocal stress tests can be evaluated with coronary CT angiography, avoiding invasive angiography if the patient has a normal test. CT, FFR, and CT perfusion are promising new tools that are now available in some centers.
* Fractional flow reserve (FFR) measures pressure difference across a coronary stenosis to determine the likelihood that it is causing myocardial ischemia. A value of 0.80, for example, means the pressure after the area of stenosis is reduced to 80% of the pressure before the area of stenosis. The magnitude of the drop in pressure correlates with the severity of myocardial ischemia. Though there is no exact point at which FFR becomes abnormal, higher values indicate non-significant stenoses, whereas lower values indicate significant lesions. In clinical trials, a cut-off point of 0.75 to 0.80 has been used.
** MET: A measure of exercise intensity based on oxygen consumption. One MET is the amount of oxygen consumed per unit of body weight during 1 minute of rest (3.5 ml/kg/min).
REFERENCES
1. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circ. 2017; 7;135(10):e146-e603. doi: 10.1161/CIR.0000000000000485.
2. Niska R, Bhuiya F, Xu J. National Hospital Ambulatory Medical Care Survey: 2007 emergency department summary. Natl Health Stat Report 2010:1-31.
3. Pope JH, Aufderheide TP, Ruthazer R, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000;342:1163-70.
4. Cury RC. President's page: coronary CT angiography as a gatekeeper to the catheterization laboratory. J Cardiovasc Computed Tomogr. 2014;8:480-2.
5. Bashore TM, Bates ER, Berger PB, et al. American College of Cardiology/Society for Cardiac Angiography and Interventions Clinical Expert Consensus Document on cardiac catheterization laboratory standards. A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001;37:2170-214.
6. Melikian N, De Bondt P, Tonino P, et al. Fractional flow reserve and myocardial perfusion imaging in patients with angiographic multivessel coronary artery disease. JACC Cardiovasc Interv 2010;3:307-14.
7. Neglia D, Rovai D, Caselli C, et al. Detection of significant coronary artery disease by noninvasive anatomical and functional imaging. Circulation Cardiovasc Imag. 2015;8 (3) .
8. Budoff MJ, Dowe D, Jollis JG, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52:1724-32.
9. Gallagher MJ, Ross MA, Raff GL, Goldstein JA, O'Neill WW, O'Neil B. The diagnostic accuracy of 64-slice computed tomography coronary angiography compared with stress nuclear imaging in emergency department low-risk chest pain patients. Ann Emerg Med. 2007;49:125-36.
10. Hachamovitch R, Di Carli MF. Nuclear cardiology will remain the "gatekeeper" over CT angiography. J Nuclear Cardiol. 2007;14:634-44.
11. Schuhbaeck A, Schmid J, Zimmer T, et al. Influence of the coronary calcium score on the ability to rule out coronary artery stenoses by coronary CT angiography in patients with suspected coronary artery disease. J Cardiovasc Computed Tomogr. 2016;10:343-50.
12. Rispler S, Keidar Z, Ghersin E, et al. Integrated single-photon emission computed tomography and computed tomography coronary angiography for the assessment of hemodynamically significant coronary artery lesions. J Am Coll Cardiol. 2007;49:1059-67.
13. Hacker M, Jakobs T, Matthiesen F, et al. Comparison of spiral multidetector CT angiography and myocardial perfusion imaging in the noninvasive detection of functionally relevant coronary artery lesions: first clinical experiences. J Nucl Med 2005;46:1294-300.
14. Tonino PA, Fearon WF, De Bruyne B, et al. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol. 2010;55:2816-21.
15. Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213-24.
16. Pijls NH, van Schaardenburgh P, Manoharan G, et al. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J Am Coll Cardiol. 2007;49:2105-11.
17. De Bruyne B, Pijls NH, Kalesan B, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367:991-1001.
18. Li J, Elrashidi MY, Flammer AJ, et al. Long-term outcomes of fractional flow reserve-guided vs. angiography-guided percutaneous coronary intervention in contemporary practice. Eur Heart J. 2013;34:1375-83.
19. Van Belle E, Rioufol G, Pouillot C, et al. Outcome impact of coronary revascularization strategy reclassification with fractional flow reserve at time of diagnostic angiography: insights from a large French multicenter fractional flow reserve registry. Circ. 2014;129:173-85.
20. Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). Circ. 2002;106:1883-92.
21. Cheitlin MD, Armstrong WF, Aurigemma GP, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography--summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). J Am Coll Cardiol. 2003;42:954-70.
22. Klocke FJ, Baird MG, Lorell BH, et al. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging). J Am Coll Cardiol. 2003;42:1318-33.
23. Greenwood JP, Motwani M, Maredia N, et al. Comparison of cardiovascular magnetic resonance and single-photon emission computed tomography in women with suspected coronary artery disease from the Clinical Evaluation of Magnetic Resonance Imaging in Coronary Heart Disease (CE-MARC) Trial. Circ. 2014;129:1129-38.
24. Bettencourt N, Chiribiri A, Schuster A, et al. Direct comparison of cardiac magnetic resonance and multidetector computed tomography stress-rest perfusion imaging for detection of coronary artery disease. J Am Coll Cardiol. 2013;61:1099-107.
25. Norgaard BL, Leipsic J, Gaur S, et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J Am Coll Cardiol. 2014;63:1145-55.
26. Cook CM, Petraco R, Shun-Shin MJ, et al. Diagnostic accuracy of computed tomography-derived fractional flow reserve : A systematic review. JAMA Cardiol 2017;2:803-10.
27. Lubbers M, Coenen A, Kofflard M, et al. Comprehensive cardiac CT with myocardial perfusion imaging versus functional testing in suspected coronary artery disease: The multicenter, randomized CRESCENT-II trial. JACC Cardiovascular imaging 2017, Dec 8.
28. Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793-801.
29. Cheruvu C, Precious B, Naoum C, et al. Long term prognostic utility of coronary CT angiography in patients with no modifiable coronary artery disease risk factors: Results from the 5 year follow-up of the CONFIRM International Multicenter Registry. J Cardiovasc Computed Tomogr. 2016;10:22-7.
30. Blanke P, Naoum C, Ahmadi A, et al. Long-Term Prognostic Utility of Coronary CT Angiography in Stable Patients With Diabetes Mellitus. JACC Cardiovascular imaging 2016;9:1280-8.
31. Johnson NP, Toth GG, Lai D, et al. Prognostic value of fractional flow reserve: linking physiologic severity to clinical outcomes. J Am Coll Cardiol. 2014;64:1641-54.
32. Arruda-Olson AM, Juracan EM, Mahoney DW, McCully RB, Roger VL, Pellikka PA. Prognostic value of exercise echocardiography in 5,798 patients: is there a gender difference? J Am Coll Cardiol. 2002;39:625-31.