
Spring 2018 - Vol. 13, No. 1
PFO Closure for Cryptogenic Stroke
Rahul Jhaveri, M.D.
The Heart Group of Lancaster General Health
INTRODUCTION
Three recently published randomized controlled trials in
The New England Journal of Medicine provide new information about closure of a patent foramen ovale (PFO) to prevent cryptogenic stroke. This article reviews the anatomy of a patent foramen ovale, the data available regarding closure, the two commonly used devices for closure, the closure procedure and its risks, the clinical testing required prior to closure, and a framework to guide patient selection for this procedure.
PFO ANATOMY AND PHYSIOLOGY
The foramen ovale is a critical feature of the fetal circulation that allows oxygenated blood to pass from the right atrium to the left atrium, thereby bypassing the uninflated lungs in utero. After birth, left atrial pressure rises above right atrial pressure, pushing the flap of the septum primum against the septum secundum and closing the foramen ovale. These tissues usually fuse over time and form the fossa ovalis, the thinnest portion of the interatrial septum. In approximately 25% of adults there is incomplete fusion of the septum primum against the septum secundum, and a patent foramen ovale persists as a one-way valve. It is usually closed, but can transiently open when right atrial pressure rises above left-sided pressure, as during a Valsalva maneuver. Some PFOs may remain open and allow bidirectional shunting. Also, atrial septal aneurysms are frequently found in association with PFOs, and these two features increase the likelihood that a PFO may cause a cryptogenic stroke.
Strokes related to a PFO arise when blood clots form on the venous side of the circulation, pass through the PFO into the left atrium, and then pass through the left heart into the cerebral circulation as a paradoxical embolism.
PFO CLOSURE
Understanding the unique features of each patient’s PFO anatomy is an important part of determining whether a PFO can and should be closed, which device to use, and which size to select. Transesophageal echocardiography (TEE) is critical in determining the amount of “rim” tissue around the PFO, and rim distances to surrounding structures, particularly the aorta and the superior vena cava.
PFO Closure Data from Randomized Controlled Trials
Six important randomized controlled trials of PFO closure have been published over the last five years that build upon observational studies showing that patients with cryptogenic stroke have an increased incidence of PFO compared with the general population.
1. The first was CLOSURE 1, a multicenter, open-label trial of PFO closure with the STARFlex device (NMT Medical) versus medical therapy, in patients between ages 18-60 with a PFO who had a cryptogenic stroke or a transient ischemic attack.
1 Medical therapy included warfarin with a target INR of 2-3, aspirin, or both. Device recipients received clopidogrel 75 mg for six months and aspirin 81 or 325 mg daily for two years. This trial showed no difference in the primary endpoint of stroke or transient ischemic attack during two years of follow up, death from any cause during the first 30 days, or death from a neurologic cause between 31 days to two years. The hazard ratio was 0.78 with a 95% confidence interval of 0.45 to 1.35, p=0.37. The STARFlex device is no longer available.
2. The second trial was PC, a multicenter trial that studied patients with a PFO and ischemic stroke, transient ischemic attack, or a peripheral thromboembolic event.
2 Patients were randomized to closure with an Amplatzer PFO Occluder or medical therapy. Patients with the closure device received aspirin for at least five to six months, and either ticlodipine or clopidogrel for one to six months. Medical therapy included antiplatelet therapy or oral anticoagulation, and all medical therapy patients received at least one antithrombotic drug.
The trial found no difference between the groups in the primary endpoints of death, nonfatal stroke, TIA, or peripheral embolism. The hazard ratio was 0.20 with a 95% confidence interval of 0.02 to 1.72, p=0.14.
3. This same Amplatzer device was then studied again in the RESPECT trial, a multicenter trial that enrolled patients between 18-60 who had a cryptogenic ischemic stroke and had a PFO identified by TEE.
3 Nine hundred eighty patients were randomized to medical therapy or closure with the Amplatzer PFO Occluder. Medical therapy included either aspirin, warfarin, clopidogrel, or aspirin with extended-release dipyridamole; patients with a device received aspirin and clopidogrel for one month, and aspirin alone for five months. There was a higher dropout rate in the medical therapy group compared with the closure group. The primary endpoint was a composite of recurrent nonfatal ischemic stroke, fatal ischemic stroke, or early death after randomization.
The intention to treat analysis of this study demonstrated no significant difference in recurrent stroke between the two groups over an average of 2.1 years. There were nine strokes in the closure group and 16 in the medical therapy group (HR 0.49, 95% confidence interval 0.22 to 1.11, p=0.08). In the as-treated cohort, however, there was a significant difference between the groups with a hazard ratio of 0.27 with a 95% confidence interval of 0.10 to 0.75, p=0.007. As discussed in the 2013 RESPECT article, three of the nine strokes that occurred in the closure group occurred without a closure device in the enrolled patient’s heart. These results generated controversy in the field. Although the Amplatzer PFO Occluder was not available for use in the United States, many operators used this data to justify off-label use of the Amplatzer Cribriform device (a similar device meant for closure of small atrial septal defects) for PFO closure in selected patients.
Recent Trials
The three more recently published randomized trials of PFO closure are: Gore REDUCE, CLOSE, and the extended follow up of RESPECT.
4. Gore REDUCE was a multi-center trial of 664 patients randomized in a 2:1 ratio to an-tiplatelet therapy plus PFO closure with the Gore HELEX or the Gore CARDIOFORM device, versus antiplatelet therapy alone.
4 Enrolled patients underwent brain imaging at baseline and at 24 months. The primary endpoints at 24 months were freedom from ischemic stroke by clinical evaluation, and the incidence of new brain infarction by imaging.
At a median follow-up of 3.2 years, clinical stroke occurred in 1.4% of the device group, and in 5.4% of the medical therapy group (HR 0.23, 95% CI 0.09 to 0.62, p=0.002). New brain infarction was also lower among the device group with a relative risk of 0.51, 95% CI 0.29 to 0.91, p=0.04). PFO closure was associated with a higher rate of atrial fibrillation (6.6%) and device complications (1.4%).
5. CLOSE was a multi-center randomized trial with three arms. Six hundred sixty-three patients between 16-60 with a history of recent stroke thought to be related to a PFO, and with either an atrial septal aneurysm or a large interatrial shunt, were randomized to PFO closure plus antiplatelet medication, to antiplatelet medication alone, or to oral anticoagulation.
5 The primary endpoint was the occurrence of stroke. After an average of 5.3 years, the PFO group did not experience any strokes, and 14 of 235 patients in the antiplatelet group experienced a stroke (HR 0.03, 95% CI 0 to 0.26, p < 0.001). There was no significant difference in the primary outcome between the antiplatelet and anticoagulation groups. Atrial fibrillation was more common in the PFO closure group (4.6%) than in the antiplatelet group (0.9%). This study is distinguished from other randomized trials by its inclusion of patients with known high-risk PFO anatomic features.
6. The third recently published randomized trial is the extended follow-up of the original RESPECT study (#3 above).
6 In the intention-to-treat analysis, and over 5.9 years of follow-up, 18 patients in the PFO group developed a recurrent ischemic stroke compared with 28 patients in the medical therapy group (HR 0.55, 95% CI 0.31 to 0.999, P=0.046). This study also demonstrated an increase in venous thromboembolism among the PFO closure group. Again, PFO closure seemed to confer additional benefit in lowering stroke risk in patients with a substantial shunt or an atrial septal aneurysm.
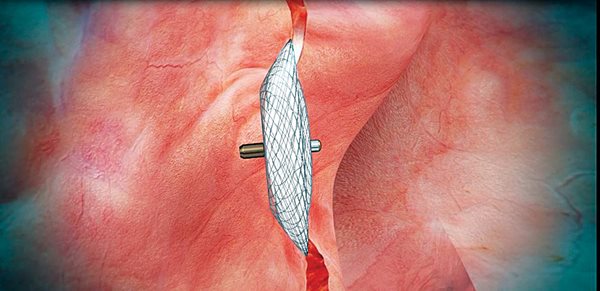
Fig. 1. Amplatzer PFO Occluder positioned across the interatrial septum. Note size of left atrial disc compared to right atrial disc.
 Fig. 2. Amplatzer PFO Occluder. Note nitinol frame with polyester fabric.
Fig. 2. Amplatzer PFO Occluder. Note nitinol frame with polyester fabric.

Fig. 3. Gore CARDIOFORM device. The hook on the left side of the device is used for device locking and delivery.
The Amplatzer PFO Occluder (Fig. 1) manufactured by St. Jude Medical is the only device approved by the FDA for transcatheter PFO closure to reduce the risk of recurrent ischemic stroke at the time of this writing. The FDA indication specifies a predominant age range between 18 to 60 years, but does not specifically exclude older patients. The device is a self-expandable double-disc device made of a Nitinol wire mesh (Fig. 2), with a left atrial disc that is smaller than the right atrial disc. The discs are connected by a waist and contain a polyester fabric. The device is MRI conditional. Implantation requires at least 9.0 mm of tissue between the PFO and the aortic root; implantation in patients with less tissue may theoretically increase the risk of device erosion and cardiac injury. Device erosion has been infrequently reported with Amplatzer Atrial Septal Occluders.
The other device that is often used for transcatheter PFO closure is the Gore CARDIOFORM Septal Occluder (Fig. 3). Use of this device for transcatheter PFO closure to reduce the risk of recurrent ischemic stroke is an off-label use as of this writing, because its intended use is for closure of ostium secundum atrial septal defects. The results of the Gore REDUCE trial may lead to an FDA approval for PFO closure in the near future. The Gore CARDIOFORM device is made of a Nitinol wire frame covered with expanded polytetrafluoroethylene (ePTFE), and it is mounted on a catheter which is delivered typically through the venous system. The risk of erosion may be lower with this device in patients with a deficient retroaortic rim, but this has not been studied systematically.
PFO CLOSURE PROCEDURE AND RISKS
PFO closure is generally performed through the femoral vein in a cardiac catheterization laboratory. It is usually a safe procedure that can be performed as an outpatient or with an overnight stay. Coronary angiography is frequently performed prior to closure if there is a suspicion of significant coronary artery disease that would warrant surgical revascularization. PFO closure requires echocardiography guidance; general anesthesia is required for TEE guidance, whereas conscious sedation can be used for intracardiac echocardiography (ICE).
Following closure, patients should be maintained on dual antiplatelet therapy (aspirin and clopidogrel) for at least one month, and aspirin monotherapy for at least five more months. After six months most patients are maintained on aspirin 81 mg alone. Patients with a history of DVT or PE are maintained on anticoagulation instead of antithrombotic therapy. Patients are advised to take antibiotic prophylaxis for six months following implantation, and to avoid strenuous activities for one-month post procedure. Trials of PFO closure have shown a small incidence (<1 %) of serious complications such as death, cardiac perforation, stroke, or major bleeding.
Three particular complications warrant further discussion: a) Several trials have shown an increased rate of atrial fibrillation (4.6 to 6.6%) following PFO closure, likely related to interaction between the device and left atrial tissue. Atrial fibrillation in this setting often resolves with time. b) Device embolization is a rare but potentially serious complication. (The Gore REDUCE trial showed a less than 1% risk of device dislocation.) Percutaneous approaches are frequently successful in recovering dislocated devices, but surgical recovery is sometimes necessary. c) Deep vein thrombosis (DVT) and pulmonary embolism (PE) may be more common following device placement. The RESPECT trial showed a PE rate of 0.41/100 patient years in the PFO closure group, versus 0.11/100 patient years in the medical therapy group, with a significant hazard ratio of 3.48 (95% CI 0.98 to 12.34, p = 0.04). The RESPECT authors suggested that patients with a cryptogenic stroke and a PFO may be at higher long-term risk for venous thromboembolism compared with the general population, and that the lower rate of anticoagulant use in the PFO closure group compared with the medical therapy group may account for this difference. Patients with a prior history of deep vein thrombosis who later develop a cryptogenic stroke, in particular warrant a discussion about the merits of lifelong anticoagulation in combination with, or instead of, PFO closure.
Clinical Testing Prior to Closure
Patients considered for percutaneous transcatheter PFO closure to reduce the risk of recurrent ischemic stroke require a thorough workup to exclude other causes of ischemic stroke. A typical workup includes MRI or CT scanning of the head to rule out small vessel or lacunar infarct; TEE to rule out other intracardiac embolic sources or aortic arch atheroma; prolonged rhythm monitoring (ideally for 30 days) to rule out atrial fibrillation; and intra and extracranial artery imaging (MRA, CTA, or contrast angiography) to assess for atherosclerotic plaque, arterial dissection, or other vascular conditions that may cause ischemic stroke. The Amplatzer PFO Occluder IFU also states that a hematologic evaluation should be done to rule out an underlying hypercoagulable state. The effectiveness of the PFO occluder has not been established in patients with a positive test for an anticardiolipin antibody (IgG or IgM), Lupus anticoagulant, beta-2 glycoprotein-1 antibodies, or a persistently elevated fasting plasma homocysteine level despite medical therapy.
Patient Selection Issues
The clinical challenge in evaluating patients with a PFO and a history of cryptogenic stroke is to determine the probability that the PFO was pathogenic and not incidental. A useful tool to address this exact question is the ROPE score, which was developed by a neurology group.
7 The score also predicts 2-year stroke/TIA recurrence rate. It is a statistically derived model which takes into account 10 clinical features that include: age, a history of diabetes, a history of hypertension, smoking status, a history of stroke or TIA, and whether a cortical infarct is present on imaging. Patients can receive a maximum score of 10 points. A patient younger than 30 with no history of hypertension, diabetes, stroke or TIA, no history of tobacco use, and a cortical infarct on imaging, would receive 10 points. A patient older than 70 with hypertension, diabetes, a prior stroke, ongoing tobacco use, and no cortical infarct on imaging would receive 0 points. The PFO-attributable fraction of cryptogenic strokes among patients with a ROPE score of 9-10 is estimated to be 88% (95% CI 83-91) with a 2-year recurrence rate of 2%. The PFO-attributable fraction of cryptogenic strokes among patients with a ROPE score of 0-3 is 0% with a 2-year recurrence rate of 20%. Although the ROPE score is not meant to be used to determine who should or should not receive a PFO closure device, it can provide a framework to guide discussions with patients.
CONCLUSIONS
The data about PFO closure to reduce the risk of recurrent ischemic stroke have evolved, and now show the benefit of closure, particularly in younger patients with an atrial septal aneurysm or a significant interatrial shunt. While rates of recurrent stroke remain low with either medical therapy or closure, many patients who have suffered a stroke and its associated debilitation, seek to lower the risk of recurrence as much as possible and may benefit from PFO closure.
An issue I encounter occasionally in practice is the younger patient with limited medical history who experienced an ischemic stroke with a pathologic-appearing PFO who has been placed on warfarin. These patients frequently are unsettled by the prospect of lifelong anticoagulation, and sometimes view PFO closure as a method to come off anticoagulation. In some of these cases, patients are indeed able to come off anticoagulation following closure, but they require a detailed explanation of the anatomic limits of PFO closure, the risk of atrial fibrillation following closure (which could require anticoagulation), and the limited randomized data that compare closure with oral anticoagulant regimens. Multiple unanswered questions remain, and a pressing one is the role of novel oral anticoagulant therapies in this population.
For now, the Amplatzer PFO Occluder is FDA approved, and is available at Lancaster General Hospital.
REFERENCES
1. Furlan AJ, Reisman M, Massaro J, et al. Closure or Medical Therapy for Cryptogenic Stroke with Patent Foramen Ovale. N Engl J Med 2012; 366:991-999. DOI: 10.1056/NEJMoa1009639
2. Meier B, Kalesan B, Mattle, H, et al. Percutaneous Closure of Patent Foramen Ovale in Cryptogenic Embolism N Engl J Med 2013; 368:1083-1091. DOI: 10.1056/NEJMoa1211716
3. Carroll JD, Saver JL, Thaler DE, et al. Closure of Patent Foramen Ovale versus Medical Therapy after Cryptogenic Stroke. N Engl J Med 2013; 368:1092-1100. DOI: 10.1056/NEJMoa1301440
4. Søndergaard L, Kasner SE, Rhodes JF, et al. Patent Foramen Ovale Closure or Antiplatelet Therapy for Cryptogenic Stroke. N Engl J Med 2017; 377:1033-1042. DOI: 10.1056/NEJMoa1707404
5. Mas JL, Derumeaux G, Guillon B, et al. Patent Foramen Ovale Closure or Anticoagulation vs. Antiplatelets after Stroke. N Engl J Med 2017; 377:1011-1021. DOI: 10.1056/NEJMoa1705915
6. Saver J, Carroll JD, Thaler DE, et al. Long-Term Outcomes of Patent Foramen Ovale Closure or Medical Therapy after Stroke. N Engl J Med 2017; 377:1022-1032. DOI: 10.1056/NEJMoa1610057
7. Kent DM, Ruthazer R, Weimar C, et al. An index to identify stroke-related vs incidental patent foramen ovale in cryptogenic stroke. Neurology. 2013 (Aug) 13;81(7):619-25. doi: 10.1212/WNL.0b013e3182a08d59. Epub 2013 Jul 17.