
Winter 2017 - Vol. 12, No. 4
TOP TIPS FROM FAMILY PRACTICE
Choosing Wisely XXII
Recommendations from the American Association of Neuromuscular and Electrodiagnostic Medicine, American Podiatric Medical Association, American Chiropractic Association
Alan S. Peterson, M.D.
Emeritus Director, Environmental and Community Medicine
Walter L. Aument Family Health Center
This is my 22nd article on “Choosing Wisely from the Board of Internal Medicine Foundation.” As previously noted, each specialty group is developing “Five or Ten Things that Physicians and Patients Should Know.”
I. RECOMMENDATIONS FROM THE AMERICAN ASSOCIATION OF NEUROMUSCULAR AND ELECTRODIAGNOSTIC MEDICINE (AANEM)
1. For isolated neck or back pain after a motor vehicle accident, needle electromyography (EMG) is unlikely to be helpful. This is especially true for those without arm pain, arm tingling, arm weakness, or arm numbness; it does not improve outcomes but does increase costs. The same is true for back pain without lower limb pain, lower limb tingling, lower limb weakness, or lower limb numbness.
2. Dermatomal Somatosensory Evoked Potentials (SEPs) are an unproven diagnostic procedure for a pinched nerve in the neck or back. Needle EMG and nerve conduction studies can be helpful to diagnose cervical or lumbar radiculopathy, but dermatomal SEP is of unproven worth for these conditions and does increase cost.
3. A four limb needle EMG/nerve conduction study (NCS) testing should not be done for neck and back pain after trauma. Four limb needle EMG/NCS is, however, rarely needed to evaluation patients for ALS, polyradiculoneuropathy, or multiple mononeuropathies.
4. Nerve conduction studies should not be done without also doing a needle EMG to test for radiculopathy, i.e. a pinched nerve in the neck or back. Nerve conduction studies alone cannot make the diagnosis; needle EMG is necessary to identify and characterize the disease process.
5. An MRI scan of the spine or brain should not be done for patients with only peripheral neuropathy (without signs or symptoms suggesting a brain or spine disorder). In most people with peripheral neuropathy (polyneuropathy), the longest nerves of the body are primarily affected (mostly in the toes and the feet, but sometimes also in the hands). There is no justification for MRI imaging of the brain or spine in these cases.
1
6. Intravenous Immunoglobulin (IVIG) should not be used in the treatment of idiopathic, length dependent, axonal polyneuropathy. IVIG is expensive, with side effects that might include severe allergic reactions, headaches, and blood clots. It is recommended for use in Guillian-Barré syndrome, chronic inflammatory demyelinating polyradiculoneuropathy, and multifocal mononeuropathy, but not other polyneuropathies.
7. B-vitamin supplements for the treatment of polyneuropathy or neuropathic pain should not be routinely used unless there is a deficiency. If a deficiency is detected, or is highly likely secondary to other medical factors (e.g., gastric bypass surgery), then B vitamins may be utilized. Excessive vitamin B-6 can lead to toxicity and worsening neuropathy, in addition to adding an unnecessary expense.
2
8. Nerve conduction studies or electromyography for muscle pain should not be performed in the absence of other abnormalities on clinical examination or laboratory testing. Muscle pain or myalgias are common; the likelihood of finding muscle disease in an individual with muscle pain but a normal neurological exam and laboratory tests, is quite low.
9. The first choice of treatment for neuropathic pain should not be opioids or narcotics. Risks related to the use of these drugs include uncontrollable sleepiness, and slowness or cessation of breathing. They can also lead to addiction and avoidable death. Opioids may be less risky when used for a short time after some surgeries or when used for pain related to deadly cancers. There are other, safer options for neuropathic pain.
10. Genetic testing for nerve and muscle diseases should not be done prior to a discussion with a physician or a genetics professional. Pretesting counseling will help patients select appropriate testing, understand the limitations of testing, anticipate potential out of pocket costs, and the effect that positive test results may have on the patient and their family.
3
II. RECOMMENDATIONS FROM THE AMERICAN PODIATRIC MEDICAL ASSOCIATION (APMA)
1. Routine use of pharmacologic DVT prophylaxis in elective foot and ankle surgery should be avoided. Implementation of prophylaxis should consider the risk of DVT in the absence of prophylaxis, and the potential adverse effects associated with the use of pharmacologic prophylaxis. The final decision should be agreed upon by the physician and the patient after a discussion of potential benefits and harms.
2. Uninfected lower extremity wounds should not be cultured, nor treated with systemic antibiotics. Uninfected wounds are contaminated with surface flora and would yield false positive culture results. Clinically uninfected wounds don’t require antibiotics; unnecessary prescriptions may have harmful side effects and lead to antibiotic resistance.
3. Patients with suspected acute rupture of the Achilles tendon do not need an MRI. MRI is expensive and can lead to treatment delays. History and physical exam findings can establish the diagnosis of acute Achilles tendon ruptures in almost all instances. MRI should be used for atypical presentations and subacute or neglected ruptures, when preoperative planning is needed. Dynamic ultrasound can be easily substituted when physicians prefer to use the rupture gap (i.e., apposition of tendon ends) as a criterion for choosing between surgery and conservative treatment.
4
4. Synthetic or donated grafts should not be utilized on diabetic foot wounds before an adequate trial of standard wound care. Most diabetic foot wounds will heal with proper wound care that includes treating any infection, ensuring that there is adequate circulation for healing, taking pressure off the wound (offloading), and debriding regularly. Synthetic or donated grafts are expensive, but might be considered if one month of standard care has not healed the wound by at least 50 percent.
5. Bone infection (osteomyelitis) in the foot does not require routine MRI. This diagnosis can be reliably established by clinical means, and/or serial plain film radiographs. MRI is particularly poor at differentiating osteomyelitis from benign postoperative marrow edema, and from marrow edema due to Charcot arthropathy.
III. RECOMMENDATIONS FROM THE AMERICAN CHIROPRACTIC ASSOCIATION (ACA)
1. Spinal imaging should not be obtained for patients with acute low back pain during the first six weeks after onset, in the absence of red flags. Red flags include a history of cancer; fracture; suspected fracture based on clinical history; progressive neurologic symptoms; infection; as well as conditions that potentially preclude a dynamic thrust to the spine, such as osteopenia, osteoporosis, axial spondyloarthritis, and tumors. Obviously, unnecessary imaging incurs cost, exposes the patient to radiation, and can result in labeling patients with conditions that are not clinically meaningful, thus creating a false sense of vulnerability and disability. Several studies have shown that the routine use of radiographs may result in worse outcomes than without their use in the care of low back pain.
5
2. Repeat imaging should not be done to monitor progress of patients with low back pain. With few exceptions (e.g. long-term management of idiopathic scoliosis), radiographic findings should not be used as outcome measures for low-back pain. Studies obviously increase costs and expose patients to more radiation, and there are no data to support a relationship between changes in alignment or other structural characteristics and patient improvement. If there is a major change in diagnosis, documented worsening of symptoms, or significant progression of disease, then repeat imaging is appropriate, but failure to respond to treatment is not an indication for repeat imaging.
3. For low back pain disorders, protracted use of passive or palliative physical therapy modalities should be avoided, unless they support the goals of an active treatment plan. “Passive therapeutic physical modalities” are defined as those interventions that require no active participation on the part of the patient. These include heat, cold, electrical stimulation, and ultrasound. Evidence demonstrates that both general physical activity (e.g., walking, jogging, biking), and specific exercise regimens, are effective in treating and preventing low-back pain and may lead to better outcomes when combined with spinal manipulation. Use of passive therapies untethered to the goal of increasing physical activity can be harmful, leading to patient inactivity, prolonged recovery and increased costs.
4. Long-term pain management should not be provided without a psychosocial screening or assessment. There is a high probability that any person with a chronic pain syndrome has a concomitant psychological disorder, most notably depression and/or anxiety. Screening tools that aid in the detection of potential depression/anxiety include: STarT Back 9 screening tool, PHQ-9 depression scale, and the Fear Avoidance Belief Questionnaire. When indicated, a referral may be most appropriate for more extensive evaluation and treatment.
5. Lumbar supports or braces should not be prescribed for long-term treatment or prevention of low back pain. They may have limited benefit in the short term, but the prolonged use of lumbar supports is not supported by the literature for the treatment or the prevention of low back pain. The currently accepted central principle of low back pain care is that the patient must engage in an active rehabilitative regimen to achieve the best outcomes.
6
TOP TIPS
CDC RECOMMENDATIONS FOR PRESCRIBING OPIOIDS FOR CHRONIC PAIN 7
Nonpharmacologic therapy and nonopioid therapy are preferred for chronic pain. Dr. Tony Ton-That, medical director of the Spine and Low Back Pain Program at LGH, discussed non-opioid management in the Winter 2016 issue of JLGH.
8 Clinical psychologist Dr. Christa Coleman provided a primer of pain psychology in the Fall 2017 issue.
9 Opioid therapy should only be considered if expected benefits for both pain and function are anticipated to outweigh risks to the patient. If they are used, they should be combined with nonpharmacologic therapy and non-opioid pharmacologic therapy, as appropriate.
Before starting opioid therapy, clinicians should establish treatment goals with all patients that are realistic regarding pain and function, and that include a discussion of how opioid therapy will be discontinued if benefits do not outweigh risks. Opioid therapy should be continued only if there is clinically meaningful improvement in pain and function.
Before starting, and periodically during opioid therapy, there should be a discussion with patients of known risks and realistic benefits, and the responsibilities of both the patient and the clinician for managing therapy.
At the beginning of opioid therapy, immediate-release, rather than extended-release/long-acting opioids should be prescribed, starting at the lowest effective dosage. Clinicians should carefully reassess evidence of individual benefits and risks when considering increasing the dosage to ≥50 morphine milligram equivalents (MME)/day. An increase in dosage to ≥90 MME/day should be resisted/avoided unless it is carefully justified.
Acute pain is often the stimulus for starting long-term use of opioids. Prescriptions should be for a quantity no greater than needed for the expected duration of the pain that requires opioids – usually three days or less – and rarely more than seven days.
Within one to four weeks of starting opioid therapy, clinicians should evaluate benefits and harms. This should continue with patients every three months, or more frequently. Tapering of the drugs to lower dosages should eventually lead to discontinuation. Clinicians need to incorporate strategies to mitigate risk, including considering offering naloxone when factors that increase risk for opioid overdose, such as history of overdose, history of substance abuse disorder, higher opioid dosages (≥50 MME/day), or concurrent benzodiazepine use are present. Concurrent use of opioid pain medication and benzodiazepines should be avoided whenever possible. I personally know of two fatal car accidents in southern Lancaster County when this combination was used.
Pennsylvania’s prescription drug monitoring program should be used to review the patient’s history of controlled substance prescriptions. Urine drug testing should be done before starting opioid therapy, and at least annually, to assess for prescribed medications as well as for other controlled prescription drugs and illicit drugs.
For patients with opioid use disorders, clinicians should offer, or arrange for, evidence-based treatment (usually medication-assisted treatment with buprenorphine or methadone in combination with behavioral therapies).
A related article from
Emergency Medicine News discusses the medicinal herb kratom,
10 which is available as a leaf, powder, or tea-like beverage. The FDA has ruled that it has no medicinal value. Opioid addicts sometimes use kratom for detoxification and to relieve withdrawal symptoms, but kratom itself can be addictive. Treatment for acute kratom toxicity is largely supportive, although naloxone may reverse some of the opioid-like effects. The initial drug of choice for seizure activity is a benzodiazepine. A comprehensive review involving pharmacology and toxicology is available.
11
LAB UPDATE ON RHEUMATOID ARTHRITIS (RA)
The 2010 Rheumatoid Arthritis Classification Criteria include two laboratory test categories.
1)
Serology assays: a) rheumatoid factor (RF); and b) anti-cyclic citrullinated peptide (anti-CCP).
2)
Acute phase reactants: a) C-reactive protein (CRP); and/or b) erythrocyte sedimentation rate (ESR).
Quantitative values of each of these should be reported. At least one test result from each category is necessary for a definitive diagnosis. Other blood tests may be ordered to help differentiate RA from other autoimmune diseases.
Immunosuppressive medications can reduce the sensitivity of the serologic and acute phase reactant assays. Other autoimmune diseases can cause low level elevation of RF. Remember that CRP and ESR indicate nonspecific inflammation and can be elevated with infections or injury, as well as other autoimmune diseases. Hepatitis C can be associated with a cryoglobulin that may have rheumatoid factor activity.
A strongly positive RF or anti-CCP assay, in the presence of more than four small joint involvements, confirms the diagnosis of RA. There is no laboratory test that, by itself, is absolutely confirmatory for RA. Guidelines state that either a CRP or an ESR must be performed even in the presence of multiple joint synovitis and a positive serologic test.
No documented drug interferences are known with these assays. RF and CRP results obtained by nephelometry (a quantitative test for immunoglobulin levels) may be falsely elevated due to lipemia, or sera containing other particulates.
ONE-THIRD OF DRUG POST-MARKET STUDIES ARE UNPUBLISHED
Investigators examined an FDA internal database to identify all post-market drug studies between 2009 and 2013 that had been identified by the agency as completed.
12 They then conducted a follow-up search to find if (and where) the studies were published. As of July 2016, 183 of the 288 post-market studies (63.5%) that met inclusion criteria were published in either the scientific literature or on the ClinicalTrials.gov website.
Of the 69 interventional clinical trials of drugs’ efficacy, 57 (86.2%) had results that were considered favorable to the trial sponsor, but these were no more likely to be published than the 12 trials with negative results.
As in previous studies, post-market study results have again been shown to be inconsistently disseminated, either through journal publication or trial registries. Publication rates for completed post-market studies required by the FDA remain relatively low.
The FDA could publish the data itself, but that would probably require new regulations. Obviously, increased sponsor commitment to publishing all clinical trials would serve to promote scientific knowledge.
TREATMENT OF RESTLESS LEGS SYNDROME IN ADULTS
Restless Legs Syndrome is estimated to affect 2.5% of U.S. adults. It affects sleep, but is primarily a neurological disease. The following (Table 1) is a practice guideline summary from the American Academy of Neurology.
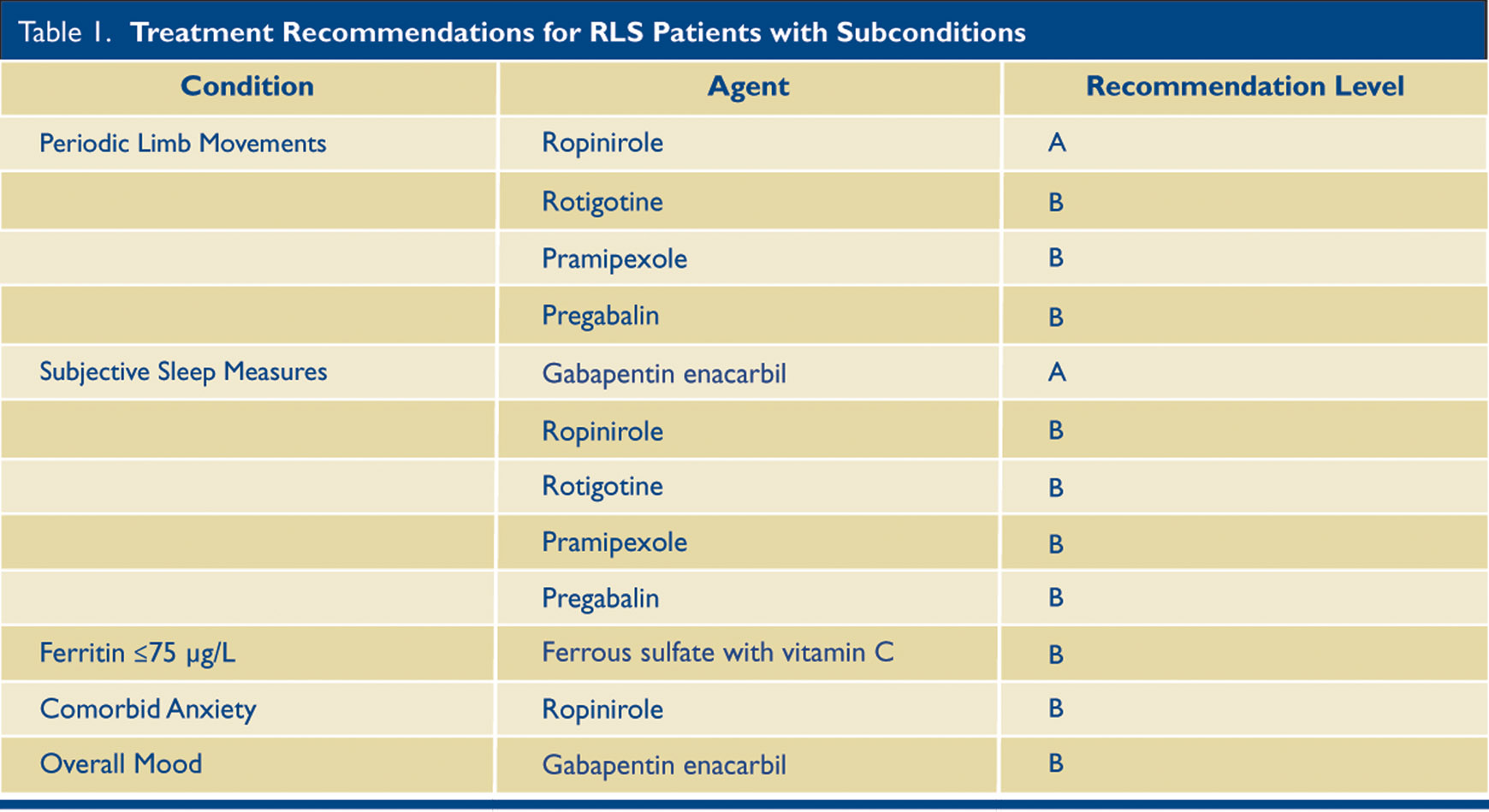
The full 72-page RLS treatment guideline is available.
13 The American Academy of Sleep Medicine has also created guidelines for restless legs syndrome (RLS) previously.
These new recommendations advocate the use of medications for adults with moderate to severe RLS, especially if symptoms disturb sleep or daytime function. (Iron insufficiency should be ruled out first, defined as a ferritin of less than 75 µg/L.)
Included below are the recommendations with subconditions. I have only included those with recommendations A and B. I have also removed the drug Cabergoline because the risk of cardiac valvopathy from high doses has made this drug infrequently used.
Pramipexole and Rotigotine, as well as Ropinirole, are dopamine agonists, which are effective in the short term for RLS, but in the longer term they can potentially worsen it. Choices of the drugs to use may be based on the patient’s age, comorbid illnesses, sensitivity to side effects, prior history of taking RLS medications, and the chief features of their individual RLS.
When nonpharmacologic approaches are desired, clinicians should consider prescribing pneumatic compression (Level B). In patients on hemodialysis with secondary RLS, clinicians should consider prescribing vitamin C and E supplements (Level B).
REFERENCES
1. England JD, Gronseth GS, Franklin G et al. American Academy of Neurology. Practice Parameter: evaluation of distal symmetric polyneuropathy: role of laboratory and genetic testing (an evidence-based review). Report of the American Academy of Neurology, American Association of Neuromuscular and Electrodiagnostic Medicine, and American Academy of Physical Medicine and Rehabilitation. Neurology 2009. Jan 13;72(2):185-92.
2. Kulkantrakorn K. Pyridoxine-induced sensory ataxic neuronopathy and neuropathy: revisited. Neurol Sci. 2014 Nov;35(11):1827-30.
3. AANEM Physician Statement. The Utility of Genetic Testing in Neuromuscular Disease: A Consensus from the AANEM on the Clinical Usefulness of Genetic Testing in the Diagnosis of Neuromuscular Disease. April 2016.
4. Singh D. Acute Achilles tendon rupture, BMJ. 2015;351:h4722.
5. National Guideline Clearinghouse. (NGC). Guideline summary: ACR Appropriateness Criteria® low back pain. In:National Guideline Clearinghouse (NGC)[Web site] Rockville (MD): Agency for Healthcare Research and Quality (AHRQ);[2016 Jan 22]. Available from:
https://www.guideline.gov/summaries/summary/49915.
6. Qaseem A, Wilt TJ, McLean RM, et al. Clinical Guidelines Committee of the American College of Physicians. Noninvasive Treatments for Acute, Subacute, and Chronic Low-back Pain: A Clinical Practice Guideline from the American College of Physicians. Ann Intern Med 2017;166(7):514-530.
7. Dowell D, Haegerich, TM, Chou R, et al. CDC guideline for prescribing opioids for chronic pain--United States, 2016. MMWR Recomm Rep. 2016 Mar 18; 65(1):1-49.
8. Ton-That T. Effective non-opioid treatment options for low back pain. J Lanc Gen Hosp. 2016; 11 (4):106-111.
9. Coleman C. A Pain Psychology Primer for Physicians. J Lanc Gen Hosp.12;3: 84-87.
10. Emergency Medicine News. March 2016--volume 38 – Issue 3 –
page 7.
11. Warner ML, Kaufman NC, Grundmann O. The pharmacology and toxicology of kratom: from traditional herb to drug of abuse. Int J Legal Med 2016;130[1]:127. doi: 10.1007/s00414-015-1279-y. Epub 2015 Oct 28.
12. Cruz, ML, Xu J, Kashoki M, et al. Publication and Reporting of the Results of Postmarket Studies for Drugs Required by the US Food and Drug Administration, 2009 to 2013. JAMA Intern Med. 2017;177(8):1207-1210. doi:10.1001/jamainternmed.2017.1313
13. Winkelman JW, Armstrong MJ, Allen RP, et al. Practice guideline summary: Treatment of restless legs syndrome in adults. Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurol. 2016. 87; 24: 2585-2593. doi.org/10.1212/WNL.0000000000003388