Fall 2017 - Vol. 12, No. 3


Davis Hughes
Obtaining Authorization for PCSK9 Inhibitors
Lessons Learned and Results of a Time Study
Tina M. Davis, MSN, CRNP, CLS, FNLA, and Joette C. Hughes, MSN, CRNP
The Heart Group of Lancaster General Health
Editor’s note: The following article describes the experiences of the Cardiology Department at LGH when they began to use new cholesterol-lowering drugs that represent an expensive departure from conventional statin therapy.
First, this experience offers lessons about the difficulties that practitioners may experience when initiating the use of any new and expensive drug. Second, the discussion of the criteria that patients must fulfill to get authorization for these specific drugs, provides guidance for practitioners trying to decide if an individual patient qualifies.
Finally, the discussion about this class of costly new drugs (about $14,500/year) brings to mind the general problem of how or whether to resist expensive drugs that have not demonstrated cost-effectiveness. Since only doctors can prescribe these expensive drugs, we control access to them. As a recent editorial in JAMA insisted, “painful as it is, draconian restrictions on access to drugs… priced for profit…may continue to be the only way medicine can send a strong signal to innovators that their future rewards are tied not just to scientific advancement but also to affordability. 1 This topic is also addressed in Dr. John Betteridge's article on IBD therapy in this issue.
INTRODUCTION
In 2015, a novel class of pharmaceuticals, PCSK9 inhibitors (proprotein convertase subtilisin/kexin type 9), became available for lipid management, and transformed the field of lipidology. Alirocumab (Praluent®) was approved by the U.S. Food and Drug Administration on July 24th, followed shortly thereafter by approval of Evolocumab (Repatha®) on August 27th.
The Lancaster General Health Physician’s Lipid Task Force anticipated that prescribing these new drugs would be bureaucratically complicated, and decided that the best approach would be to limit their prescription to specialists in cardiology and endocrinology. This proved to be a wise decision, since the barriers to obtaining authorization for these medications ultimately included restrictions imposed by outside agencies on who can prescribe them. Indeed, many insurance companies will only pay for PCSK9 inhibitors when they are prescribed by cardiologists, lipidologists, or endocrinologists.
CRITERIA FOR DRUG APPROVAL
After the Heart Group Preventive Cardiology and Apheresis Clinic developed a process for the authorization of PCSK9 inhibitors, Alirocumab was prescribed for the first patient on 8/24/15. The first lesson learned was the importance of identifying appropriate adult patients for therapy. Such patients must meet all three of the following criteria for approval:
1) a diagnosis of atherosclerotic cardiovascular disease with or without homozygous or heterozygous familial hypercholesterolemia,
2) prior treatment with a high intensity statin (Rosuvastatin or Atorvastatin) with or without Ezetimibe, or clearly documented intolerance of statins and Ezetimibe,
3) an LDL cholesterol level above goal.2,3
The difficulty with the first criterion is proving the diagnosis of heterozygous familial hypercholesterolemia without genetic confirmation. Most FH patients are diagnosed based on clinical and biochemical features. A definite diagnosis can be made if the patient meets specific criteria of the Simon Broome or Dutch Lipid Network Criteria scoring systems, but some of the patient’s personal and familial information is often unavailable, making it difficult to establish a definite diagnosis with the scoring tools. Occasionally, a payer will accept the phenotypic diagnosis of heterozygous familial hypercholesterolemia based on the National Lipid Association’s definition, which requires only an LDL level ≥ 190 mg/dL and a family history of premature coronary artery disease.4
Analysis of the Heart Group population that has been prescribed PCSK9 inhibitors reveals their ages ranged from 45 to 87 years, with 51% female and 49% male. Out of 172 patients, 153 (91%) had atherosclerotic cardiovascular disease, 124 (73%) had heterozygous familial hypercholesterolemia, and 108 (64%) had both diagnoses. Of the 172 patients, 123 reported statin intolerances, and 19 (11%) had both diagnoses plus statin intolerance.
Before prescribing a PCSK9 inhibitor for patients who presented with statin failure, they were evaluated for tolerance of a retrial of statins with or without Ezetimibe. Fortunately, of the 123 statin intolerant patients, 72 (59%) tolerated a statin when re-challenged, yet they still required additional PCSK9 therapy to achieve their LDL goal. Further evaluation revealed that 82/172 (48%) patients were managed with statins alone, 73 (42%) took Ezetimibe alone, and 43 (25%) patients needed statins plus Ezetimibe. Finally, laboratory data revealed that the range of LDL levels at the time of prescribing was 87-354 mg/dL, with LDL goals < 100 for primary prevention and < 70 for secondary prevention.5 Although our patient population was like others across the nation, our high percentage of patients with familial hypercholesterolemia is a unique local finding due to a known founder population of Amish reported in 2010.6,7
THE APPROVAL PROCESS
Once an appropriate patient was identified, the next step was to determine their payer and identify the pharmacy benefits manager, to understand their criteria for approval. Obtaining authorization was complicated by the inconsistency of criteria among payers. In addition, payers utilized multiple specialty pharmacies, which required a variety of authorization forms. Furthermore, in 2015, many payers seemed unprepared for the onslaught of prescriptions, had no policies in place, and employed authorization representatives who were unprepared for their role in the process. As a result, payers frequently denied approval even though they were provided proper documentation that their clients met the payer’s approval criteria.
The considerable time required to obtain authorization for PCSK9 inhibitors created staffing problems in our office. Authorization forms were completed and submitted by the nurses, who were required to make frequent phone calls to payers, pharmacies, primary care offices, and patients. The time needed to achieve approvals was also increased by appeals and peer-to-peer reviews.
LESSONS LEARNED FROM A TIME STUDY
To fully understand the time commitment needed for authorization of these novel drugs, the Heart Group carried out a time study on medication authorization during a two-month period from 11/9/16 to 01/31/17. Nurses documented the time taken to approve medications in three different areas of the institution: a) two PCSK9 inhibitors by the Prevention Clinic; b) Entresto and seven pulmonary hypertension medications by the Congestive Heart Failure Clinic; and c) general cardiac-related medications by the Prescription Department.
The total time spent among the three departments for medication authorization averaged 87.23 hrs/week. Surprisingly, the Prevention Clinic dedicated 38.16 hrs/week to the two PCSK9 inhibitors, while the Congestive Heart Failure Clinic and the Prescription Department needed 14.17 hrs/week and 34.5 hrs/week respectively for authorization of a greater number of medications. It was also interesting that the time employed in getting authorization for these novel drugs did not diminish within the eight-week study period, which indicated that gaining experience with the process did not reduce the time required. The results of this time study prompted The Heart Group to hire two medical assistants in the Prescription Department to assume the responsibilities of novel drug authorizations that were becoming a burden on the nursing staff.
The next lesson was that documentation is critical to obtaining approval. While nurses completed the authorization forms, medical providers were responsible for identifying the correct patient and documenting the approval criteria. A letter of medical necessity created from an Alirocumab template letter was placed in Epic as a smart phrase. The medical provider was advised to complete the letter the day the PCSK9 inhibitor was prescribed, so that it could accompany the initial authorization request in anticipation of a quicker approval. If the request was denied, the initial letter could be updated and used as an appeal letter. Though it took more time in the beginning for the provider to generate a letter, it saved a great deal of time overall if – as often happened – the initial application was denied.
Another barrier in the approval process was poor documentation of treatment failures with statins and/or Ezetimibe. Frequently, there was simply no documentation in the patient’s chart. Details of specific statins attempted, doses and frequency, adverse reactions, and resolution of symptoms could not be identified. It became necessary to investigate the primary care provider’s chart to obtain a history of documented medication intolerance. The last resort for documentation of failed medication was a review of medications with the patient’s pharmacy personnel who maintain a 10-year record of prescribed medications. Despite these efforts, there were times when no documentation was recovered, and the patient had to be re-challenged to prove and document the failure of statins.
RESULTS
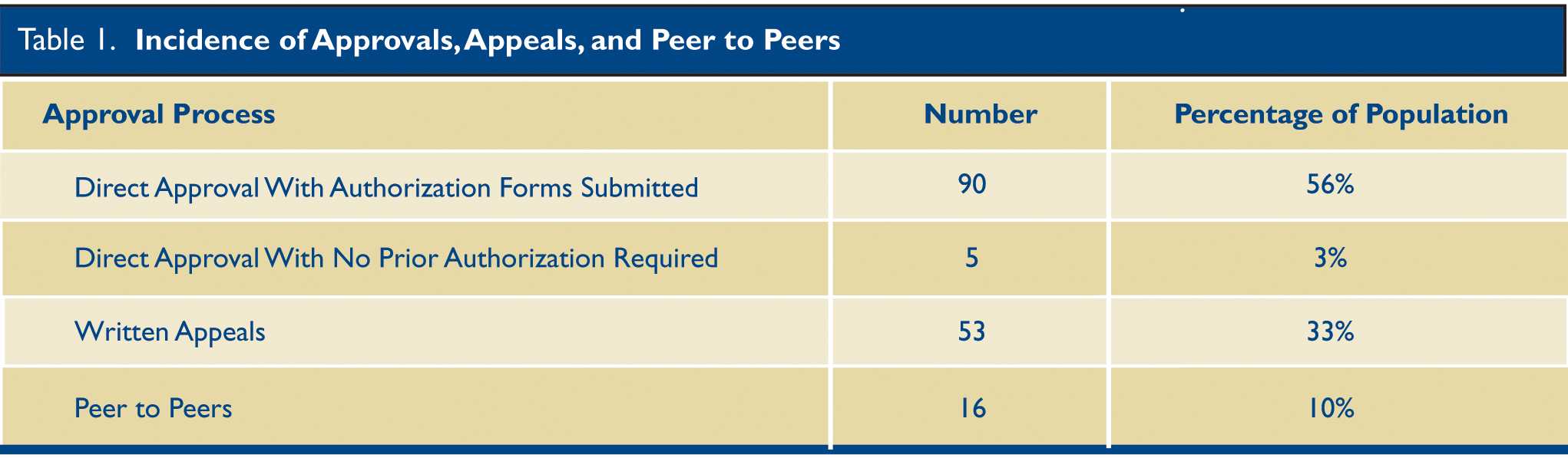
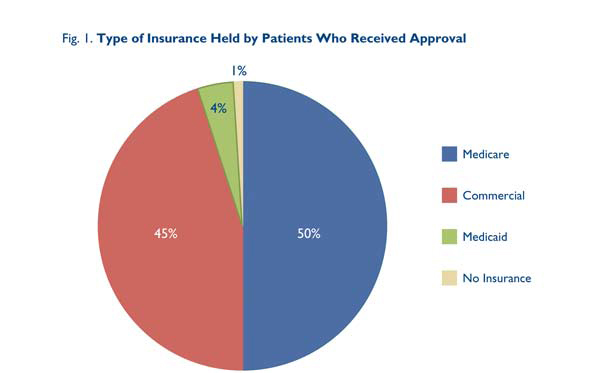
The authorization process was analyzed for 161 Heart Group patients who received approvals. (Table 1.) Five were approved without any pre-authorization; 85 were approved after submission of the initial authorization forms without any further steps; 55 were denied initially but were approved on appeal; 13 required peer-to-peer reviews for approval; and three were denied despite appeals. One half of the population was covered by Medicare, 45% by commercial Insurance, 4% by Medicaid payers, and 1% had no coverage. (Fig. 1.)
 The
The
Heart Group team achieved a 98% approval rate for PCSK9 inhibitor therapy, far surpassing the national average of 22-46% in the Medicare population, and 12-27% in the population with commercial coverage.8,9
COST
Financial burden is the final barrier that must be addressed, as the retail list price for PCSK9 inhibitors is approximately $14,600 annually.10 Initially, the objective was to prescribe PCSK9 inhibitors for those who met the criteria, and then obtain financial assistance if necessary. Patients covered by commercial insurance are able to utilize co-pay cards for great affordability, but Medicare and Medicaid patients are unable to use co-pay cards. The cost for most Medicare patients is $200-500/month, with a few plans reducing the cost below $100 monthly. However, Medicaid co-pays are usually below $20 monthly.
Cost is discussed with all patients, but they may not recognize that this is the principal barrier preventing them from obtaining PCSK9 therapy. Fortunately, financial assistance plans are available in Pennsylvania including PACE and PACE NET programs for low-income individuals. In 2015 and 2016, the Patient Assistance Network Foundation was able to assist some patients who met the qualifications, but in 2017 very little funding has been available from that program. An alternative for patient’s taking Repatha is Amgen’s Safety Net program for those with very high co-pays or no insurance, who meet the criteria. Communication about cost is critical at the beginning of the process to set realistic expectations for each patient. Only then can an informed decision be made whether to seek approval.
CONCLUSIONS
In summary, multiple lessons were learned while maneuvering the approval process for PCSK9 inhibitors. Patient advocacy is imperative in obtaining this lipid lowering medication that is medically necessary. Identifying the appropriate patient, and understanding the payer’s coverage criteria, lay the foundation for greater success. Next, employing and empowering staff that are passionate and committed to the process is essential. Adequate time will be mandatory for staff to retrieve and submit documentation proving the patient meets the criteria required by payers for approval. Finally, communicating clearly about cost, and setting realistic expectations, will help the patient and provider negotiate the process more effectively. The authorization process may seem tedious and time consuming, but the benefits of obtaining approval of PCKS9 inhibitors for very high-risk patients may be manifested best by fewer cardiovascular events according to the Fourier outcomes trial.11 Better cardiovascular health for our patients is well worth these efforts.
REFERENCES
1. Mark DB, Schulman KA. PCSK9 inhibitors and the choice between innovation, efficiency, and affordability. JAMA. 2017;318(8):711-712. doi:10.1001/jama.2017.8907
2. Praluent prescribing information. Sanofi US and Regeneron Pharmaceuticals https://www.praluent.com/what-is-praulent?moc=pluco2500pps&utm_source=google&utm_medium=cpc&utm_campaign=2016_G_DTC_Branded&utm_content=Drug Information_Praluent Information_E&utm_term=praluent pi&gclid=CPHag_ncq9ECFdRtgQodDhUNNA&gclsrc=ds
3. Repatha Prescribing Information. Amgen, Inc. https://www.repatha.com/?WT.z_co=A&WT.z_in=LDL&WT.z_ch=PDS&WT.z_st=Site1&WT.z_mt=BM&WT.z_pdskw=rapatha&WT.z_se=G&WT.srch=1&WT.z_prm=Branded%202016_Misspellings%20Broad&WT.mc_id=A_LDL_PDS_G_Branded%202016_Misspellings%20Broad_BM_rapatha
4. Goldberg AC, Hopkins PN, Toth PP, et al. Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients: clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5:133–140.
5. Hughes, J, Davis, T, Estrella, L. PCSK9 Use in a high risk population – Familial Hypercholesterolemia. Poster presentation at ACC 2017, Washington D.C., March 2017.
6. Shen, H, Damcott CM, et al. Familial defective apolipoprotein B-100 and increased low-density lipoprotein cholesterol and coronary artery calcification in the old order Amish. Arch Intern Med. 2010;170(20):1850-1855.
7. Andersen, Lars. Familial defective Apolipoprotein B-100 in Lancaster County and beyond. The Journal of Lancaster General Hospital. 2016;11(1):15-17.
8. Davis, T, Hughes, J, Estrella, L. Team approach to prescribing success for PCSK9 Inhibitors. Journal of Clinical Lipidology. 2017;11(3): 810. Poster presentation at the National Lipid Association Scientific Sessions in Philadelphia, May 2017.
9. PCSK9 inhibitors: payer dynamics. Symphony Health Solutions website. http://science.sciencemag.org/content/232/4746/34.long
10. Pollack, A. “New drug sharply lowers cholesterol, but it’s costly.” The New York Times. July 24, 2015.
11. Sabatine, MS, Giugliano, RP, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. NEJM. 2017;376:1713-1722.