
Spring 2017 - Vol. 12, No. 1
Update on Acute Stroke Management
Murray Flaster, M.D., Ph.D.
Stroke Medical Director, Lancaster General Health
INTRODUCTION
Over the last 20 years, there have been major advances in the care of patients with acute stroke due to progress in imaging and therapeutic technology, coupled with randomized control trials (RCTs) that have been difficult to undertake yet have been crucially important. Although medical progress is a continuum, we can and should recognize the most noteworthy milestones. This paper discusses the impact of two key milestones in acute stroke care.
I. INTRAVENOUS TPA
Twenty-one years ago marked the beginning of a new era in the care of acute ischemic stroke when the NINDS (National Institute of Neurological Disorders and Stroke) published the results of its trial of recombinant tissue plasminogen activator (tPA).
1 Prior to 1995, when neurologists like me received a call from an emergency room physician or an admitting physician regarding a patient with acute stroke, we only had to ask: “Did the CT scan of the head show hemorrhage?” If the reply was “no,” then a typical recommendation might be “give the patient an aspirin and I will see them in the morning.” After the December 1995 publication of the NINDS stroke trial, treatment with tPA revolutionized care of acute ischemic stroke.
Any adult with a presumed ischemic stroke with a non-trivial deficit could now potentially be treated within three hours of onset of symptoms. If a CT scan of the head showed neither acute hemorrhage nor ischemia that was already advanced, and a few contraindications were excluded or corrected, tPA was administered at 0.9mg/kg over one hour (a dose 20% less that the dose given for acute MI and approved years earlier). Other than the prerequisite CT of the head, no other imaging was required and the etiology of the ischemic stroke need not be specified. Despite a 5%-6% risk of intracerebral hemorrhage, patients treated with tPA within the three-hour window were twice as likely to have an excellent outcome. Notably, favorable outcomes were inextricably linked to time from onset of stroke to initiation of treatment; when treatment was initiated within 60 minutes of onset the odds of an excellent outcome (no deficit or no functional limitation) were more than 3.5 times better than in controls. By three hours the odds of an excellent outcome dropped to about 1.2 (Fig. 1). In other words, “as soon as possible” treatment is demonstrably superior to “just in time” treatment.

Fig. 1. When the NINDS outcomes odds ratios (ORs) are displayed as a function of time, early treatment yields a likelihood of 3.5 or better of an excellent outcome (mRS < 1 — modified Rankin Scale, an index of functional independence). By three hours excellent outcomes are only slightly more frequent than in patients not treated with tPA. “Just in time” just isn’t good enough.
Secondary analysis of the results of the NINDS stroke study showed treatment was effective in the three major subtypes of stroke: cardioembolic, large vessel, and lacunar infarction, each accounting for about one quarter of the ischemic stroke population. To the surprise of many, tPA was effective in small vessel stroke where the a priori presence of thrombus, at least in the view of many experts, was less certain. There was a caveat, however, for patients with occlusion of the major intracranial vessels: the internal carotid artery terminus, the middle cerebral artery stem or M1 and the basilar artery. Though these patients with potentially the most catastrophic strokes could be successfully treated, they were less likely to enjoy major recovery.
Since that publication, it has taken years to disseminate this type of swift, effective, and safe care of acute stroke. Starting with a handful of academic and large community hospitals, the new protocols eventually reached health systems in nearly all settings, urban and rural, national and international.d the results of its trial of recombinant tissue plasminogen activator (tPA).
2 To accomplish this objective, hospitals had to develop systems of care that included emergency medical services (EMS), emergency department (ED) physicians, technologists in radiology and the laboratory, radiologists, neurologists, stroke telemedicine networks, internists and critical care physicians. Finally, community education was crucial. As a result, the use of an IV thrombolytic in appropriate patients is now relatively commonplace, and ED to treatment (“door-to-needle”) times have improved. Current national guidelines call for all door-to-needle times to average less than 60 minutes, while door-to-needle times under 45 minutes should be achieved in 50% of patients.d the results of its trial of recombinant tissue plasminogen activator (tPA).
3,4
In the years since the seminal publication there have been additional RCTs, and reports of clinical experience in case series and stroke registries. These have led to a few important refinements in care, and extension of treatment to special populations.
5 The risk of clinically significant intracranial hemorrhage has proved to be considerably lower than early observations suggested, even as low as 1.8% in one very large database.
6 With this observation, we are encouraged to treat patients with milder deficits, or those showing some clinical improvement since onset of symptoms.
Guidelines regarding the time window for treatment have been extended from 3 hours to 4.5 hours for most patients.
7 Trial data show that within the later 3.0 to 4.5-hour window, thrombolysis benefits patients without clearly evolving stroke on CT scan as well as patients not taking any anti-coagulant, and does not increase the risk of bleeding.
8 Note, however, that the benefit is modest at best, and it does not extend to patients over 80 years of age, nor to diabetics with a history of prior ischemic stroke. The mantra “Time is Brain” remains paramount; later treatment times still offer opportunities to treat, but expected outcomes never equal those available to patients treated earlier.
II. MECHANICAL THROMBECTOMY
It was clear from the early days of thrombolytic therapy that it offered less benefit to the many patients with large clot burdens in major intracranial vessels. Treatment of these patients was marked by occasional success, but results were inconsistent until early 2015 when four RCTs were reported that primarily employed stent-retrievers. Identification of eligible patients depended largely on the utilization of CT angiography, a noninvasive technique that has evolved over the last 15 or 16 years and has sufficient speed and accuracy to determine which patients harbor occlusions of large intracranial vessels.
9 Results of these four seminal trials were sufficiently compelling that in June 2015 an American Heart Association/American Stroke Association Guidelines were issued
10 with the following recommendations: adult patients with occlusion of the intracranial internal carotid artery or proximal M1 were candidates for mechanical thrombectomy if strokes were sufficiently severe (NIH Stroke Scale > 6) and CT of the head showed only modest ischemic changes (Alberta Stroke Program Early CT Score or ASPECTS greater than 5). Groin puncture should be initiated within six hours of symptom onset, and patients should also first be treated with IV tPA according to current guidelines, if not contraindicated.
Meta-analysis was recently completed of five major stent-retriever trials encompassing 1,287 patients, of whom 634 were randomized to mechanical thrombectomy, while controls received IV tPA only.
11 Both treatment groups had severe strokes with a baseline NIHSS of 17. The individual RCTs differed in a number of details, including admission criteria and — to a lesser extent — study protocols. Still, the collaborators felt there were sufficient data to permit the pooling of individual data and reaching important conclusions. At 90 days, patients who were functionally independent (defined as a modified Rankin Scale (mRS) of 0 through 2) numbered 46% in the mechanical thrombectomy groups, compared with 26.5% in the control groups. A shift toward a better outcome was about 2½ times more likely in the interventional arm, common odds ratio (cOR) = 2.49 (confidence interval 1.76 to 3.53, P < 0.0001). Other measures of benefit were statistically and clinically significant as well.
When analyzed by overall shift in functional outcome, benefit was seen in select subgroups: patients greater than 80 years of age (cOR = 3.68, confidence interval 1.95 to 6.92) and in patients randomized five or more hours after symptom onset (cOR = 1.76, confidence interval 1.05 to 2.97). Further studies are needed to establish benefit in smaller clinical subgroups. For instance, there were too few patients aged 50 and under to permit statistical inference, and the same was true of patients with occlusion of the more distal M2 branch of the middle cerebral artery. Basilar artery occlusions remained too infrequent to enter into trials for now. But the overall results of these five trials are on the whole robust, and cannot be ignored.
An important conclusion from further meta-analysis of the 1,287 patients described above shows clearly that time remains a crucial factor to treatment success: for patients with groin puncture at three or fewer hours cOR of some functional benefit was 2.79 (confidence interval 1.96 to 3.98), but for patients with groin puncture between three and six hours the cOR for some functional benefit was reduced to 1.98 (confidence interval 1.30-3.00).
12 Treatment initiated between six to eight hours no longer yielded a statistically significant benefit, with a calculated treatment boundary of seven hours and 17 minutes marking the limit of statistical utility. We can conclude that for successful treatment with mechanical thrombectomy, “time is brain” remains as true as with IV tPA.
Effective stroke programs need to concentrate on minimizing time to treatment and not on statistically derived treatment windows alone. Until we can freeze the ischemic brain in place, treatment windows are boundaries, not goals.
To that end, minimizing time from onset of stroke to definitive treatment needs to be organized in a way that minimizes all delays, whether in stroke recognition, total transport time, effective diagnostic imaging, essential laboratory studies, and accurate clinical evaluation. Many systems of care current currently rely upon two staged evaluations, which would include a primary stroke center and then a secondary or tertiary stroke center capable of mechanical thrombectomy. Transport often involves a combination of ground and air transport. This approach has the advantage of encompassing large populations but has the disadvantage of prolonging time to treatment.
CASE STUDIES
Following are five cases treated in Lancaster County recently that illustrate the current diagnosis and treatment of acute stroke. Four of the five cases have a very favorable outcome. This is admittedly a biased representation, but we wish to illustrate not just what is theoretically possible, but what acute stroke therapy can already achieve in the real world. We also suggest future steps that would improve acute stroke outcomes.
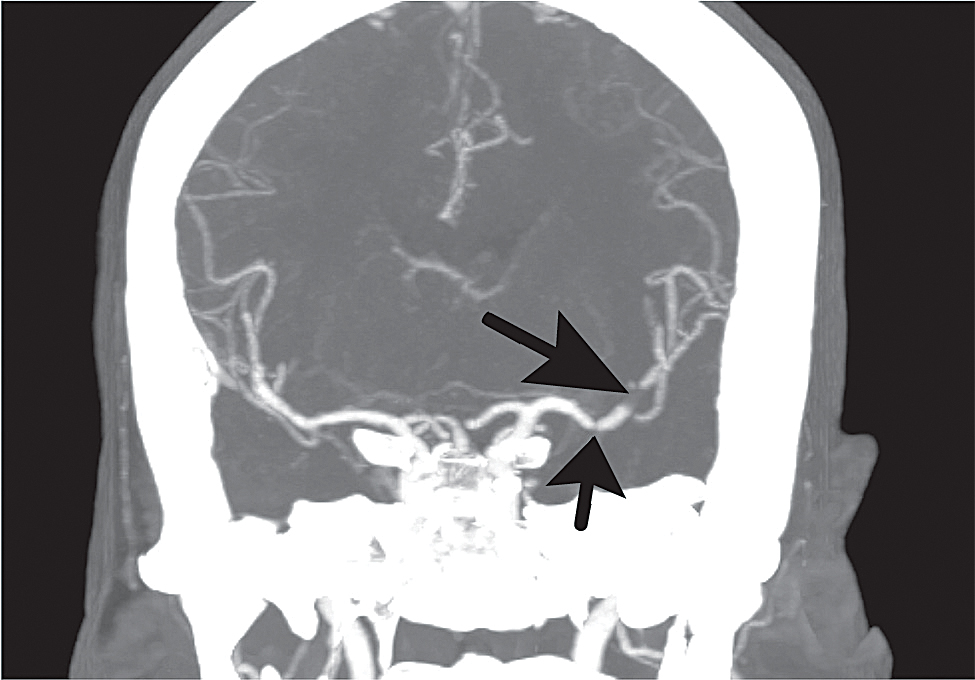
Fig. Case 1. CT angiogram of the head, coronal MIP (multi-planar image projection) showing a thrombus occluding the distal M1 and an M2 branch (long arrow) and a smaller thrombus partially occluding the M1 more proximally (short arrow).
Case 1
A 63-year-old woman was recovering uneventfully from coronary artery bypass surgery one week earlier when she developed sudden aphasia and right hemiparesis while taking her morning medications. Her husband called EMS, which brought the patient to the LGH ED 75 minutes after the onset of symptoms. Evaluations by the emergency department physician and the stroke neurologist found global aphasia and dense right hemiparesis, with an NIH stroke scale of 16 — indicating severe impairment. Past medical history was significant for hypertension, hyperlipidemia, and recent ST-elevation MI. She was a nonsmoker but had a strong family history of cardiac disease. CT of the head was unremarkable but CT angiogram demonstrated thrombus in the middle cerebral artery, mid and distal M1. (Fig. Case 1) Because of her recent surgery, she was not a candidate for intravenous thrombolytic. Sixty minutes later she was emergently transferred by helicopter to Penn where mechanical thrombectomy was performed 4½ hours from onset. She recovered full movement within hours and by the following morning language function had fully normalized. She has been in an excellent state of health since, and without neurological deficit (NIHSS 0, mRS 0).
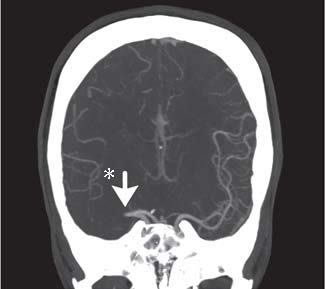
Fig. Case 2. Coronal MIP showing proximal right MCA complete occlusion (arrow). Notice that the distal MCA vasculature on the right shows far fewer contrast containing vessels than the comparable area on the left (*).
Case 2
A 31-year-old woman was at an outdoor party when she developed sudden left-sided weakness. EMS was called and a stroke emergency protocol was initiated. She was evaluated by the ED physician immediately upon arrival and sent to CT scan. She was seen very shortly thereafter by the stroke neurologist. She was mildly somnolent with obvious left facial weakness, unable to move either the left upper or lower extremity. Mild left-sided neglect was present. Sensory extinction was present but there was no gaze preference. CT of the head showed a very questionable right middle cerebral artery dense vessel sign, but CT angiography demonstrated a completely occluded proximal right middle cerebral artery. (Fig. Case 2) The patient had a mild headache. There were no contraindications to thrombolysis, and Intravenous tPA was administered 59 minutes after onset of symptoms. Arrangements for helicopter transfer for mechanical thrombolytic therapy at Penn were initiated, but the patient's weakness began to improve dramatically 25 minutes after administration of IV tPA, and transfer was cancelled. She continued to improve and her deficits resolved fully without the need for further intervention. She was discharged from the hospital NIHSS 0, mRS 0.
Her past medical history was significant for headache with occasional migrainous features. She was a cigarette smoker, and she had resumed birth control pills some months prior to her stroke. She had a history of two normal, full-term pregnancies and no miscarriages. She was believed to have a hypercoagulable state secondary to hormonal therapy and cigarette smoking. No genetic or acquired defect in the coagulation pathway was found.
Case 3
A 52-year-old gentleman with a history of obesity and hypertension developed acute right-sided weakness and slurred speech just after walking his dog. His wife called EMS, which found right-sided weakness and dysarthria and declared an out-of-hospital stroke alert. On arrival in the ED he was taken immediately for a head study and CT angiography. CT of the head showed neither a dense vessel sign,
* nor evidence of early or prior ischemia. CT angiography showed widely patent middle cerebral and vertebra-basilar arteries. Examination demonstrated a completely flaccid right arm, weak right lower face, a right lower extremity which was barely antigravity, and mild dysarthria and right hemisensory loss. NIHSS summed to 10, a significant deficit. Although blood pressure in the field was 220/100, it lowered spontaneously to 178/92 on site.
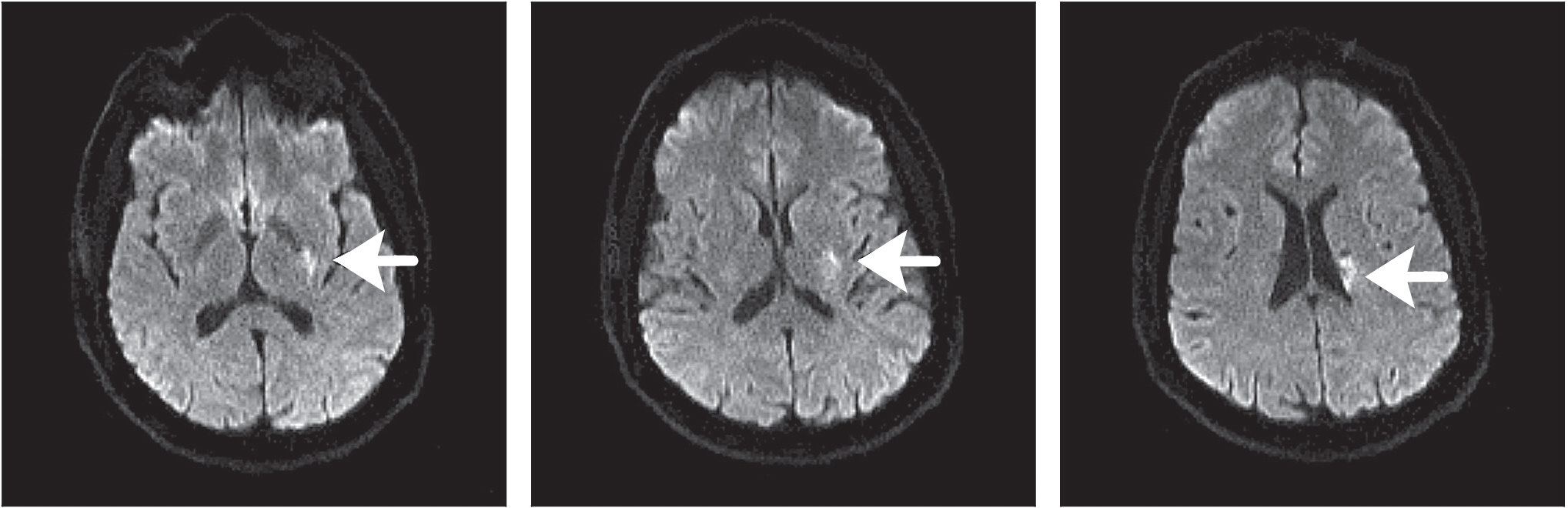 Fig. Case 3. Across the top are three consecutive images that are echo-planar diffusion weighted, and demonstrate left lenticulostriate injury (arrows). The image at lower left is an Apparent Diffusion Coefficient (ADC) map confirming diffusion is restricted in the same area (arrow).
Fig. Case 3. Across the top are three consecutive images that are echo-planar diffusion weighted, and demonstrate left lenticulostriate injury (arrows). The image at lower left is an Apparent Diffusion Coefficient (ADC) map confirming diffusion is restricted in the same area (arrow).
There were no contraindications to intravenous tPA, and thrombolytic treatment began 44 minutes after ED arrival (74 minutes after onset of symptoms). He began to improve 20 minutes after treatment started. Dysarthria disappeared, power in both extremities was normal, and the facial droop became quite subtle. MRI of the brain demonstrated a left lenticulostriate infarct (Fig. Case 3). No large vessel atherosclerotic disease was apparent in the cervical or intracranial vasculature. Echocardiography demonstrated mild concentric left ventricular hypertrophy as the only abnormality. LDL was quite elevated at 158 mg/dL. The patient was discharged home on the third hospital day with low-dose aspirin, clopidogrel, and a maximum dose of atorvastatin. He was without deficits and without any need for rehabilitation (NIHSS 0, mRS 0).
Case 4
A 67-year-old left-handed man developed sudden left-sided weakness that caused him to slide to the ground while at home. He did not lose consciousness or injure himself. The family called EMS, which noted his left-sided weakness and called in a pre-hospital stroke alert. During transport, they recorded improvement in his initial profound weakness. In the emergency department, he was evaluated by the ED physician and the stroke neurologist who found no difficulties with speech or language, and no facial effects. He had pure left-sided weakness, could barely maintain his left arm against gravity, and had a slightly weak and ataxic left leg. NIH stroke scale was 4, an estimate of mild to moderate disability. CT of the head was unremarkable while CT angiography of the head and neck demonstrated no intracranial large vessel abnormalities. Both internal carotid arteries had disease at their origins and irregular plaque in the vessels, but only the left internal carotid artery was stenosed. Moderate aortic arch atherosclerotic plaque was also present.
There were no contraindications to intravenous thrombolytic and the patient received IV tPA 43 minutes after arrival in the ED. Within 30 minutes of initiation of thrombolytic therapy the patient began to improve dramatically, and by hospital discharge two days later he had no neurological deficit.
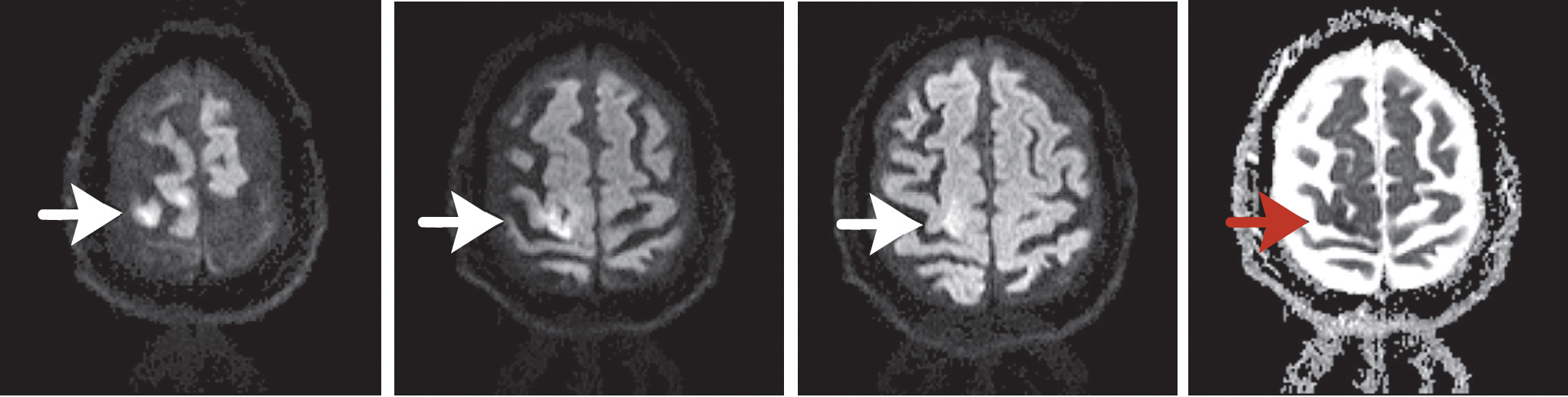
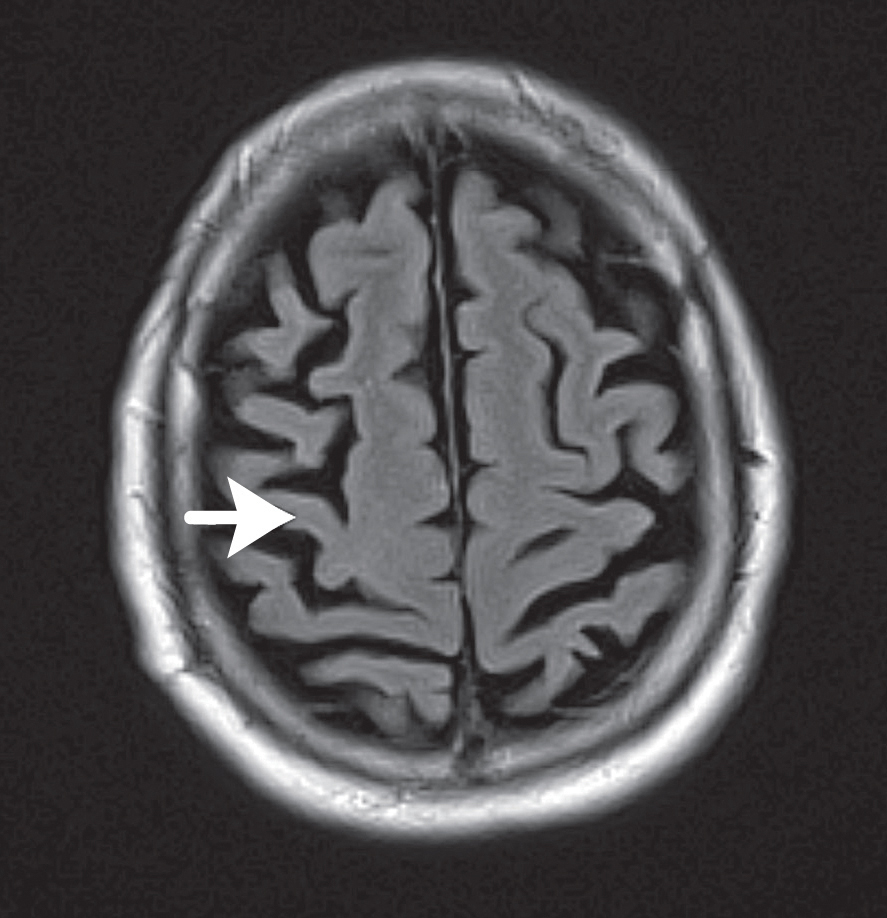 Fig. Case 4. At the top, from left, are three successive echo-planar diffusion-weighted images of cerebral cortex. The three arrows point to the ischemic lesion. The panel at the top right shows the corresponding ADC map, which confirms diffusion restriction (red arrow). At lower left is a T2 FLAIR image. This confirms the location of the lesion confined almost entirely to the motor strip (primary motor cortex). The central sulcus is just below.
Fig. Case 4. At the top, from left, are three successive echo-planar diffusion-weighted images of cerebral cortex. The three arrows point to the ischemic lesion. The panel at the top right shows the corresponding ADC map, which confirms diffusion restriction (red arrow). At lower left is a T2 FLAIR image. This confirms the location of the lesion confined almost entirely to the motor strip (primary motor cortex). The central sulcus is just below.
MRI of the brain demonstrated acute diffusion restriction involving the left motor strip (Fig. Case 4), presumably from an artery-to-artery embolism. Echocardiography during hospitalization demonstrated a mildly hypokinetic infero-septal wall but no definite cardiac source of emboli. Telemetry showed no abnormalities.
The patient had a lengthy past medical history, which included adult-onset diabetes, coronary artery disease and congestive heart failure, carotid artery stenosis that included remote right carotid endarterectomy, peripheral vascular disease, and gout. The etiology of his stroke was certainly embolic, probably from the previously operated right carotid. He was discharged on aspirin, clopidogrel, and as high a dose of statin as he tolerated. Arrangements were made for further cardiac rhythm monitoring as an outpatient to better exclude episodic atrial fibrillation.
Case 5
A 66-year-old woman became suddenly aphasic and right hemiparetic in her kitchen in the late evening. Her husband called EMS, which declared a stroke alert very soon after they arrived. After arrival in the ED, exam revealed global aphasia and moderate weakness of face, arm and leg on the right, NIHSS 10. CT of the head done immediately after arrival showed no acute ischemic changes. A dense vessel sign was not present. CT angiography of the head and neck showed a widely patent left M1 but occlusion of the left M2. The patient received IV tPA 59 minutes after arrival in the ED, a little over two hours from onset of symptoms. In the next half hour, power and comprehension improved, and the patient did well until the following morning when she began to develop fluctuating weakness and worsening comprehension. At first her symptoms improved with an IV fluid bolus and lying flat, but unfortunately she again deteriorated. The initial brain MRI showed fairly limited diffusion restriction, but an MRI 24 hours later showed clear extension of the ischemic injury (Fig. Case 5).
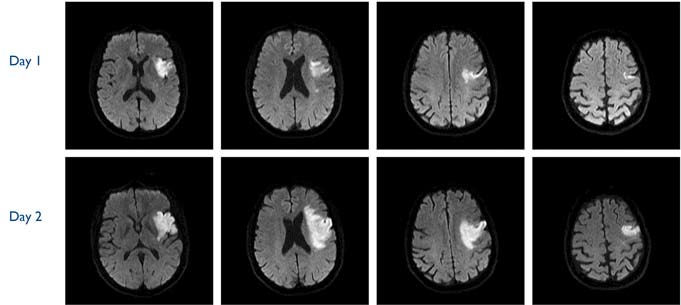 Fig. Case 5. Top row comprises four consecutive diffusion-weighted images demonstrating relatively minor diffusion injury to the superior division of the left MCA. The bottom row of images obtained about 24 hours later shows obvious extension of the ischemia. This may reflect a combination of recanalization followed by reocclusion together with failure of collateral flow.
Fig. Case 5. Top row comprises four consecutive diffusion-weighted images demonstrating relatively minor diffusion injury to the superior division of the left MCA. The bottom row of images obtained about 24 hours later shows obvious extension of the ischemia. This may reflect a combination of recanalization followed by reocclusion together with failure of collateral flow.
The patient eventually stabilized and went to rehabilitation where she improved modestly with respect to comprehension and lower extremity power. She has continued to slowly improve since. There is a history of hypertrophic cardiomyopathy but no evidence of atrial fibrillation until weeks after her stroke.
DISCUSSION OF CASES
The first case demonstrates the effectiveness of mechanical thrombectomy in a patient who was not eligible for intravenous thrombolytic because of her recent cardiac surgery. A CT angiogram done in the ED pinpointed the lesion and made clear the only available path to improvement. This patient’s deficit resolved entirely, a dramatic benefit that can only be obtained in the presence of very good collateral flow.
The second case demonstrates the effectiveness of IV tPA when given early enough, even when the lesion is in a major intracranial vessel. The patient enjoyed complete resolution of symptoms. There is currently a lively ongoing debate regarding whether patients with M1 occlusions should go directly to mechanical thrombectomy. This case clearly suggests otherwise, providing treatment can be delivered early.
The third patient had a
lacunar or small vessel infarction, for which IV thrombolysis is currently the only available effective treatment. The patient had a large enough deficit that — in the absence of CT angiography — might have been assumed to be due to occlusion of M1 or M2. Again, early treatment is key to an excellent outcome. It is believed that thrombolytic therapy works in lacunar stroke because there is thrombus present, most likely at the origin or ostium of the arteriole, which is typically a 100-200µ diameter branch vessel off the MCA, vertebral, or basilar artery. Quite frequently a micro-atheroma may be present at the vessel ostium, helping to explain not only the efficacy of thrombolytic therapy, but also the efficacy of statins in lacunar stroke.
Case 4 is an example of artery-to-artery embolism. Even small lesions to the motor strip can cause devastating disability. In this case the deficit was modest, it was treated early, and the patient’s deficits resolved entirely. Again, early treatment was key to a successful outcome.
Case 5 illustrates the instability of major intracranial branch lesions. The patient’s M2 occlusion did not qualify for mechanical thrombectomy under current guidelines, and she improved considerably with initial therapy. Unfortunately, many patients will subsequently worsen under these circumstances, and at the moment we cannot predict their clinical course.
CONCLUSIONS
Ischemic stroke is heterogeneous. Cases 1 and 5 were cardioembolic; Case 3 was a case of small vessel disease in a patient with hypertension and hyperlipidemia; Case 4 was an artery-to-artery embolism in a patient with carotid disease treated with endarterectomy years earlier but with recurrence of internal carotid artery unstable atherosclerotic plaque; and Case 2 involved a young person with a presumed hypercoagulable state. Such cases are relatively rare.
Early treatment leads to remarkably good results, but it depends upon heightened community awareness and excellent coordination between the EMS, the ED, and the stroke team. Rapid and accurate diagnosis facilitates appropriate treatment. Mechanical thrombectomy is now an important part of acute stroke treatment.
Overall, it is estimated that a population of about 1 million people can support a clinically and economically effective stroke program capable of timely intravenous and mechanical therapies. Currently, Lancaster County and immediately adjoining areas have a population of 1.2 million people that are within a 40-minute drive of downtown Lancaster. This suggests that further development of acute stroke capabilities locally would benefit everyone here, and in the surrounding areas, by drastically reducing time-to-treatment, eliminating costly and time-consuming helicopter transport, and removing the added burden that family suffers when treatment is distant.
* “Dense vessel sign” or “hyper-dense arterial sign” may be seen on non-contrast CT scan in about one quarter of patients with acute ischemic stroke. Specificity and sensitivity are far below CT angiography results and inter-observer reliability is not good.
REFERENCES
1. Stroke Study Group. (1995). Tissue plasminogen activator for acute ischemic stroke. N Engl J Med, 333, 1581-1588.
2. Fonarow, G. C., Zhao, X., Smith, E. E., Saver, J. L., Reeves, M. J., Bhatt, D. L., ... & Schwamm, L. H. (2014). Door-to-needle times for tissue plasminogen activator administration and clinical outcomes in acute ischemic stroke before and after a quality improvement initiative. JAMA, 311(16), 1632-1640.
3. Jauch, E. C., Saver, J. L., Adams, H. P., Bruno, A., Demaerschalk, B. M., Khatri, P., ... & Summers, D. R. (2013). Guidelines for the early management of patients with acute ischemic stroke. Stroke, 44(3), 870-947.
4. Target; Stroke — American Stroke Association
www.strokeassociation.org/targetstroke
5. Demaerschalk, B. M., Kleindorfer, D. O., Adeoye, O. M., Demchuk, A. M., Fugate, J. E., Grotta, J. C., ... & Saposnik, G. (2016). Scientific Rationale for the Inclusion and Exclusion Criteria for Intravenous Alteplase in Acute Ischemic Stroke. Stroke, 47(2), 581-641.
6. Mazya, M., Egido, J. A., Ford, G. A., Lees, K. R., Mikulik, R., Toni, D., ... & Ahmed, N. (2012). Predicting the risk of symptomatic intracerebral hemorrhage in ischemic stroke treated with intravenous alteplase. Stroke, 43(6), 1524-1531.
7. Del Zoppo, G. J., Saver, J. L., Jauch, E. C., & Adams, H. P. (2009). Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator. Stroke, 40(8), 2945-2948.
8. Hacke, W., Kaste, M., Bluhmki, E., Brozman, M., Dávalos, A., Guidetti, D., ... & Schneider, D. (2008). Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. New England Journal of Medicine, 359(13), 1317-1329.
9. Menon, B. K., & Demchuk, A. M. (2011). Computed tomography angiography in the assessment of patients with stroke/TIA. The Neurohospitalist, 1(4), 187-199.
10. Powers, W. J., Derdeyn, C. P., Biller, J., Coffey, C. S., Hoh, B. L., Jauch, E. C., ... & Meschia, J. F. (2015). 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment. Stroke, 46(10), 3020-3035.
11. Goyal, M., Menon, B. K., Van Zwam, W. H., Dippel, D. W., Mitchell, P. J., Demchuk, A. M., ... & Donnan, G. A. (2016). Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. The Lancet, 387(10029), 1723-1731.
12. Saver, J. L., Goyal, M., van der Lugt, A., Menon, B. K., Majoie, C. B., Dippel, D. W., ... & Cardona, P. (2016). Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: A meta-analysis. JAMA, 316(12), 1279-1288.