Spring 2017 - Vol. 12, No. 1
Autologous Breast Reconstruction
Following Breast Cancer
Joseph M. Serletti, M.D., FACS
Chief, Division of Plastic Surgery, Penn Medicine
Martin J. Carney, B.S.
Clinical Research Fellow, Division of Plastic Surgery, Penn Medicine
Jason M. Weissler, M.D.
Clinical Research Fellow, Division of Plastic Surgery, Penn Medicine
David S. Warsaw, D.O., MBA
Chief, Division of Plastic Surgery, Lancaster General Health
BACKGROUND AND SIGNIFICANCE
One of every eight women in the United States develops breast cancer in her lifetime.
1 As a result, roughly 2.5 million women in the U.S. are survivors of breast cancer, and this number is expected to increase dramatically over the next decade.
1
In women with breast cancer, the selection of the initial surgical procedure is influenced by the cancer’s stage at the time of diagnosis. With early stage cancer, nearly 58% of women undergo breast-conserving surgery, and 36% undergo mastectomy. With more advanced breast cancer, if surgery is performed, mastectomy is carried out in 58% of patients, and breast-conserving therapy is used in 14%.
2 Thus, mastectomy continues to play a major role in the treatment of breast cancer, and in most cases, offers the chance for longterm survival. In addition to women who undergo mastectomy, a substantial percentage of those who have breast conservation therapy are unhappy with their ultimate cosmetic result.
3
There are also other reasons for an increasing need for reconstruction. With the advent of risk stratification by genetic testing for BRCA1 and BRCA2, an increasing number of women are electing prophylactic mastectomy. Also, the frequency of contralateral prophylactic mastectomy is increasing dramatically in patients who undergo mastectomy for unilateral disease.
4, 5 Thus, studies over the past 20 years have shown a distinct trend toward increased use of contralateral and bilateral prophylactic mastectomy.
6
The mastectomy defect can be devastating both physically and psychologically, and numerous studies have documented a significant improvement in self-confidence and mental health following breast reconstruction.
7,8,9 Reported rates of reconstruction vary widely, but a study in two major American cities reported that 42% of mastectomy patients underwent reconstruction.
10 As reported by the American Society of Plastic Surgeons, in 2015 over 100,000 patients received breast reconstruction, a 35% increase since 2000. Although less than 20% of these cases were autologous free flap procedures, these operations are more prevalent at high volume breast reconstruction centers, such as the University of Pennsylvania.
The public’s awareness of this option must still be improved, as only 23% of women know the wide range of available options for breast reconstruction.
10
RECONSTRUCTION TECHNIQUES
Options for reconstruction include alloplastic techniques, such as insertion of a tissue expander/implant, or autologous reconstruction with free flaps. Alloplastic techniques have been more commonly utilized nationally as they avoid the need for technically challenging microsurgery. A recent study by our institution demonstrated that although there was a lower complication profile for alloplastic reconstructions, they were followed by higher rates of secondary breast procedures.
11 Additionally, we have shown a higher two-year success rate using free flap reconstruction, a significantly lower rate of unplanned surgical revisions, and lower cost.
12
Importantly, no single procedure is favored in all circumstances, and patients clearly benefit from individual selection of the best procedure. Patients who see a plastic surgeon are now often well informed about their options in advance. The discussion between surgeon and patient about an overall plan for reconstruction must be an open dialogue that involves both listening and education.
Autologous Reconstruction
Perhaps the most monumental contribution to autologous breast reconstruction was the introduction of the pedicled transverse rectus abdominis myocutaneous (TRAM) flap procedure by Hartrampf et al. in 1982.
13 This technique remains the most common method of autologous breast reconstruction. Though free tissue transfer for breast reconstruction was first reported by Fujino et al. in 1976, the technique did not become popular in the United states until the 1980s and 1990s.
14 Subsequently, use of free tissue transfer has increased because of greater familiarity with microvascular techniques. Examples of these approaches include the free TRAM flap, the deep inferior epigastric perforator (DIEP) flap, and the superficial inferior epigastric artery (SIEA) flap.
Although these autologous approaches remain less common than alloplastic reconstructions, using a patient’s own tissue has some distinct advantages. Autologous reconstruction has the benefit of replacing “like with like,” which in turn contributes to an improved feeling of restoration of the self after mastectomy.
15 Methods of using abdominal donation have the added benefit of the concomitant abdominoplasty, which many women find appealing. The disadvantage is the rare possibility of catastrophic complete loss of the flap, necessitating further surgery. Our institution has previously published a 2% take-back rate in an analysis of 2260 total free flaps reviewed.
16 In addition, autologous reconstruction is a more involved operation, with longer recovery time and potential for donor-site morbidity.
After mastectomy, autologous reconstruction can be safely performed either immediately or in a delayed fashion. Immediate reconstruction has several advantages, since patients benefit from needing only one operation and most surgeons find immediate reconstruction easier to perform due to the predictable mastectomy skin flap envelope. Precise planning of the location of the skin island can be designed on the abdomen before transfer, thus improving the efficiency and precision of the operation. Delayed reconstruction requires reevaluation of the skin flaps, which are often scarred and less compliant. The mastectomy scar should be excised completely as scarred or irradiated skin can result in inadequate breast ptosis and poor symmetry over time. Fig. 1 demonstrates the standard TRAM flap design, while Fig. 2 depicts a pre and post-operative photograph of a patient undergoing unilateral reconstruction. When reattachment of vessels is appropriate, the tissue can be transferred as a free flap with anastomosis to the internal mammary artery. Alternatively, the tissue can be pedicled, leaving the vasculature attached and rotating the tissue up from the abdomen. A pedicled TRAM flap rotates along a tissue axis connected to the flap’s blood supply, and is placed into the correct position on the chest wall.
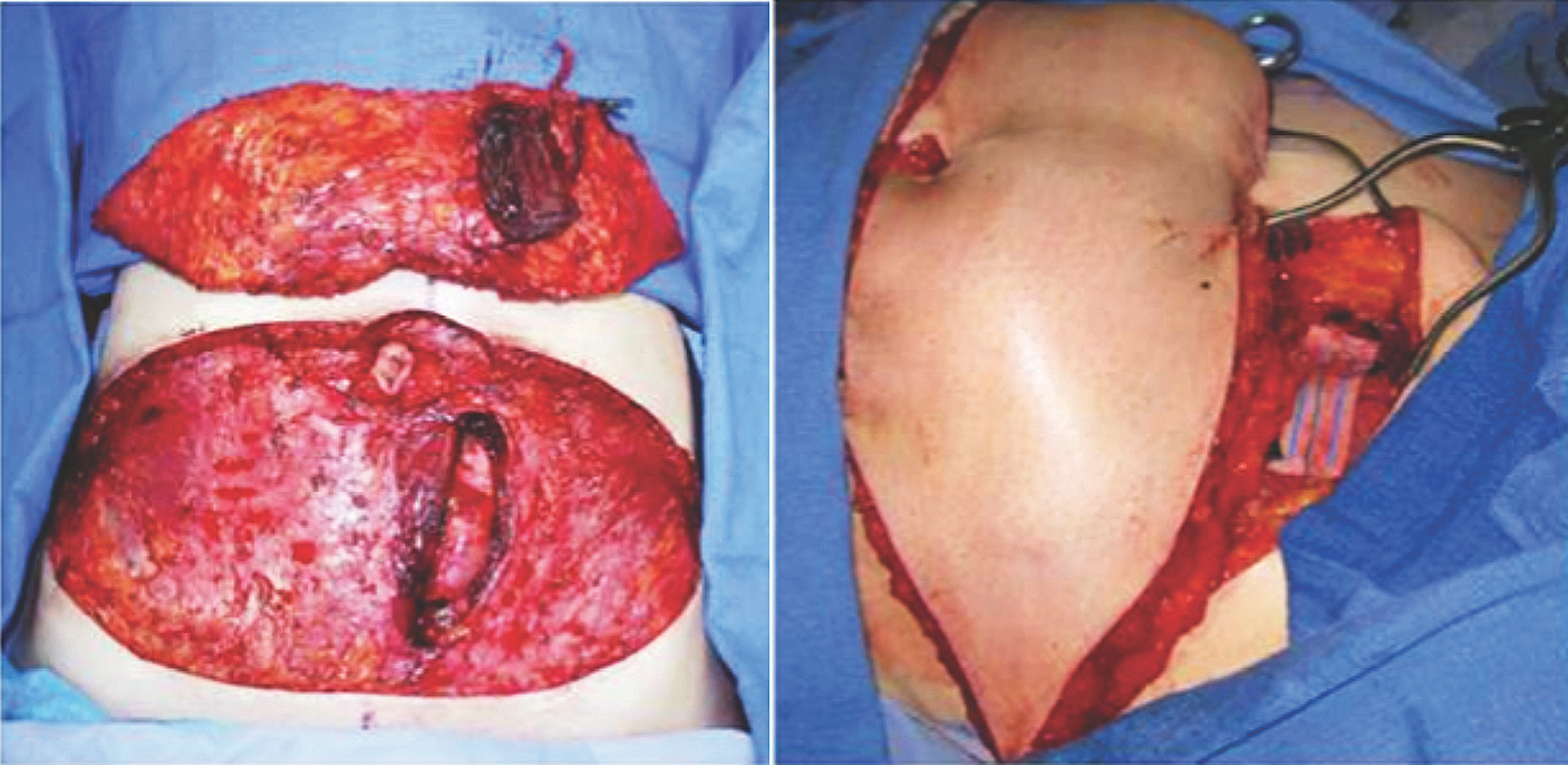 Fig. 1. (Left) The standard TRAM flap design. (Right) The free flap anastomoses are performed to the internal mammary vessels. Penn Plastic Surgery photos.
Fig. 1. (Left) The standard TRAM flap design. (Right) The free flap anastomoses are performed to the internal mammary vessels. Penn Plastic Surgery photos.
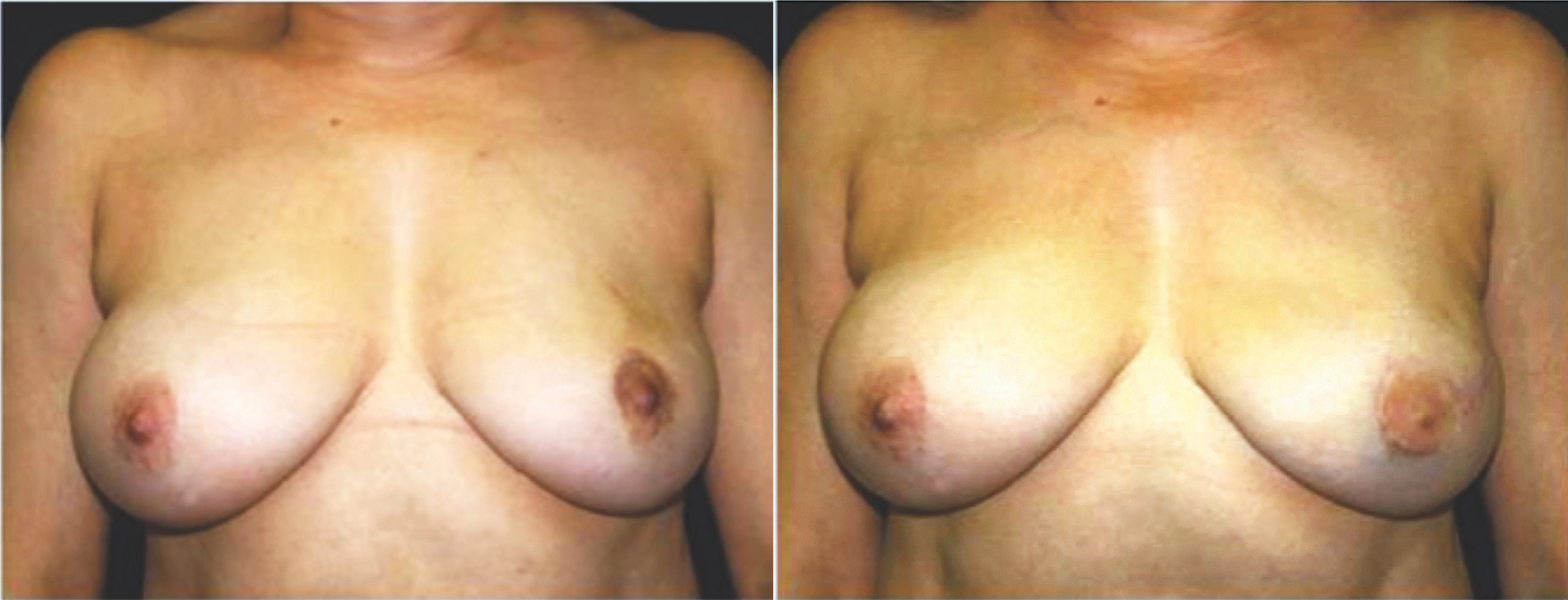
Fig. 2. (Left) Preoperative photograph of a patient with left breast cancer. (Right) The patient underwent a left free TRAM flap procedure with nipple reconstruction and tattooing. Penn Plastic Surgery photos.
Nipple-Areola Complex Reconstruction
After reconstruction of the breast mound, many women opt to have the nipple-areola complex recreated as a final step in restoring body image. There are many available techniques, including grafts from skin, buccal mucosa, labia minora or majora, thigh, buttocks, groin, upper eyelids, or earlobes. Although the technique varies, the principles remain the same: to provide color, texture, size, and projection that are all in line with the patient’s aesthetic wishes. Many of the earlier methods suffered from loss of projection over time and difficulty with color matching and stability. The most common method used today consists of local skin flap rearrangement to create texture and projection followed by tattooing for color (Fig. 3). Nipple-areola complex (NAC) reconstruction is usually performed more than 3 to 4 months after mound reconstruction. This allows for complete healing of the flap, and ensures breast symmetry, an important prerequisite before recreating a NAC.
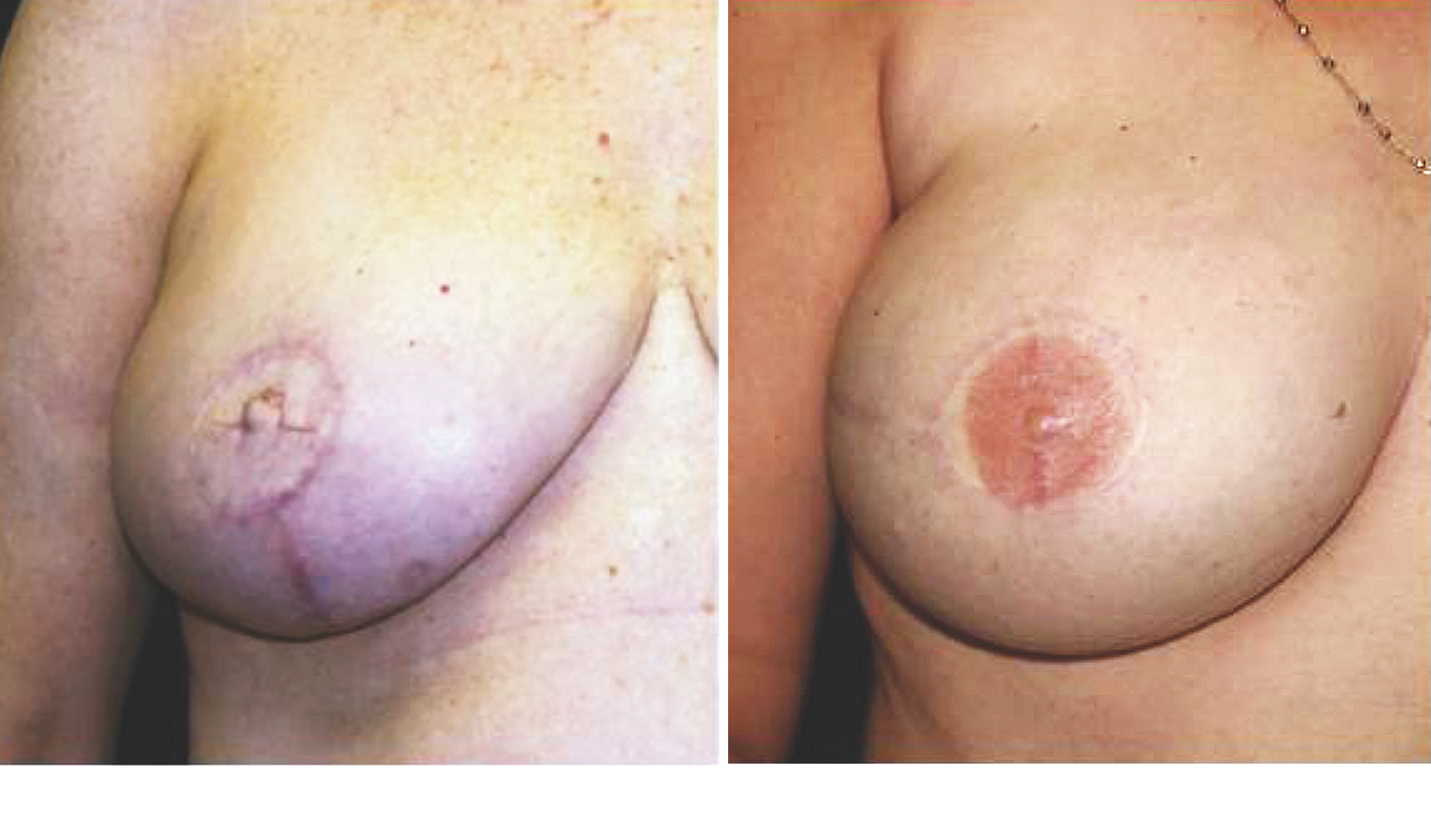 Fig. 3. (Left) This patient underwent autologous breast reconstruction followed by nipple-areola complex reconstruction. She has yet to undergo tattooing for color. (Right) This patient had a similar presentation and operative intervention. She has completed the tattooing process for color. Penn Plastic Surgery photos.
Nerve Coaptation
Fig. 3. (Left) This patient underwent autologous breast reconstruction followed by nipple-areola complex reconstruction. She has yet to undergo tattooing for color. (Right) This patient had a similar presentation and operative intervention. She has completed the tattooing process for color. Penn Plastic Surgery photos.
Nerve Coaptation
Restoration of breast sensation following breast reconstruction is an evolving part of the reconstruction paradigm. Although spontaneous recovery of sensation has been reported following mastectomy, surgeons are advocating innervated reconstruction using neurotization at the time of autologous reconstruction. This technique dates back to the early 1990s, but has yet to be rigorously tested in a large prospective randomized control trial. Fig. 4 demonstrates the graft and nerve coaptation connecting the transferred abdominal tissue to the chest wall. Restoring sensation to the reconstructed breast via nerve coaptation is an invaluable adjunctive procedure on the cutting edge of breast reconstruction.
 Fig. 4. Diep flap breast reconstruction with nerve conduit neurotization (arrow) to enhance sensation in the reconstructed breast. Penn Plastic Surgery photo.
Fig. 4. Diep flap breast reconstruction with nerve conduit neurotization (arrow) to enhance sensation in the reconstructed breast. Penn Plastic Surgery photo.
Follow-Up
In unilateral reconstruction, patients will need to continue routine mammography of the contralateral breast. Because of the possibility of recurrence, patients should be instructed to continue self-examination of the reconstructed breast. Mammography of the reconstructed mound has been shown to be less sensitive for detecting malignant lesions when compared with the native breast. Nonetheless, there have been reports of calcifications being detected by mammography, allowing diagnosis of a recurrence that was not palpable.
17,18,19 Currently, however, routine mammography of the reconstructed breast mound is not considered standard of care.
CONCLUSIONS
Breast reconstruction is becoming increasingly popular and is important to many women following treatment of breast cancer. There are many options available to the patient, but ultimately which technique is used should be based on surgeon familiarity, hospital resources, and the patient’s wishes. All can be offered with a high degree of patient satisfaction and a low rate of complications.
REFERENCES
1. De Angelis R, Tavilla A, Verdecchia A, et al. Breast cancer survivors in the United States: Geographic variability and time trends, 2005-2015. Cancer 2009; 115:1954–1966.
2. DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64: 252-271
3. Wang HT, Barone CM, Steigelman MB, et al. Aesthetic outcomes in breast conservation therapy. Aesthet Surg J. 2010; 28:165–170.
4. Tuttle TM, Jarosek S, Habermann EB, et al. Increasing rates of contralateral prophylactic mastectomy among patients with ductal carcinoma in situ. J Clin Oncol. 2009; 27:1362–1367.
5. Stucky CC, Gray RJ, Wasif N, et al. Increase in contralateral prophylactic mastectomy: Echoes of a bygone era? Surgical trends for unilateral breast cancer. Ann Surg Oncol. 2010;17(Suppl 3):330–337.
6. Cemal, Y., Albornoz, C. R., Disa, et al. A paradigm shift in U.S. breast reconstruction: Part 2. The influence of changing mastectomy patterns on reconstructive rate and method. Plast Reconstr Surg 2013; 131:320e-326e.
7. Sarwer DB. Psychological Aspects of Reconstructive and Cosmetic Plastic Surgery: Clinical, Empirical, and Ethical Perspectives. Philadelphia: Lippincott Williams & Wilkins; 2006.
8. Guyomard V, Leinster S, Wilkinson M. Systematic review of studies of patients’ satisfaction with breast reconstruction after mastectomy. Breast 2007; 16:547–567.
9. Alderman AK, Kuhn LE, Lowery JC, et al. Does patient satisfaction with breast reconstruction change over time? Two-year results of the Michigan Breast Reconstruction Outcomes Study. J Am Coll Surg. 2007; 204:7–12.
10. Morrow, M., Li, Y., Alderman, A. K., et al. Access to breast reconstruction after mastectomy and patient perspectives on reconstruction decision making. JAMA Surg 2014; 149:1015-1021.
11. Fischer, JP, Fox, JP, Nelson, JA, et al. A Longitudinal Assessment of Outcomes and Healthcare Resource Utilization After Immediate Breast Reconstruction-Comparing Implant- and Autologous-based Breast Reconstruction. Ann Surg 2015; 262:692-699.
12. Fischer, JP, Wes, AM, Nelson, JA, et al. Propensity-matched, longitudinal outcomes analysis of complications and cost: comparing abdominal free flaps and implant-based breast reconstruction. J Am Coll Surg 2014; 219:303-312.
13. Hartrampf CR, Scheflan M, Black PW. Breast reconstruction with a transverse abdominal island flap. Plast Reconstr Surg. 1982; 69:216–225.
14. Fujino T, Harashina T, Enomoto K. Primary breast reconstruction after a standard radical mastectomy by a free flap transfer: Case report. Plast Reconstr Surg. 1976; 58:371–374.
15. Tønseth KA, Hokland BM, Tindholdt TT, Abyholm FE, Stavem K. Quality of life, patient satisfaction and cosmetic outcome after breast reconstruction using DIEP flap or expandable breast implant. J Plast Reconstr Aesthet Surg. 2008; 61:1188–1194.
16. Mirzabeigi, MN, Wang, T, Kovach, SJ, et al. Free flap take-back following postoperative microvascular compromise: predicting salvage versus failure. Plast Reconstr Surg 2012; 130:579-589.
17. Lee JM, Georgian-Smith D, Gazelle GS, et al. Detecting nonpalpable recurrent breast cancer: The role of routine mammographic screening of transverse rectus abdominis myocutaneous flap reconstructions. Radiology 2008; 248:398–405.
18. Helvie MA, Bailey JE, Roubidoux MA, et al. Mammographic screening of TRAM flap breast reconstructions for detection of nonpalpable recurrent cancer. Radiology 2002; 224:211–216.
19. Edeiken BS, Fornage BD, Bedi DG, et al. Recurrence in autogenous myocutaneous flap reconstruction after mastectomy for primary breast cancer: US diagnosis. Radiology 2003; 227:542–548.