
Summer 2016 - Vol. 11, No. 2
Fecal Microbiota Transplantation:
Indications, Effectiveness, Our Experience, and Future Directions
Paul Allegretti, D.O.
Lancaster Gastroenterology Inc.
INTRODUCTION
There has been an alarming increase in the incidence and severity of Clostridium difficile infection (CDI)1 since around the turn of the century (See Fig. 1). Cases of CDI nearly doubled from 98,000 in 1996 to 178,000 in 2003, and have been paralleled by an increased unadjusted case-fatality rate of 1.2% in 2000 to 2.3% in 2004. Mortality currently approaches 30,000 deaths annually. Of equal concern is an increased incidence of community-associated CDI which is being reported in populations previously considered low risk, including peripartum women, patients with cirrhosis or inflammatory bowel disease, and healthy individuals without a history of antibiotic use.
1

Current antibiotic treatment regimens do not offer satisfactory cure rates. After 10-14 days of treatment with Flagyl or Vancomycin, an initial episode of CDI has a recurrence rate of 20%-25%, which increases to 40% after one recurrence, and further increases to 60%-65% after two recurrences. Current treatment regimens for recurrent Clostridium difficile infection (RCDI) require prolonged regimens of Vancomycin or administration of the new oral tablet Fidaxomicin. These regimens are quite costly and require tapers that can last many months with marginal efficacy. The significant number of patients who experience additional CDI relapses have even more limited treatment options. Other interventions that have been reported, but with little or no data to support efficacy, include intravenous immunoglobulin (IVIG), rifaximin, nitazoxanide, and probiotics.2,3
Against this backdrop, Fecal Microbiota Transplantation has been gaining attention and mainstream acceptance due to its impressive efficacy in treating RCDI.
BACKGROUND
Fecal microbiota transplantation (FMT), i.e. the introduction of a fecal suspension from a healthy screened donor into the gut of a diseased patient, was actually introduced 1700 years ago by a Chinese scientist named Ge Hong4 who administered human fecal suspensions orally to treat patients with food poisoning or severe diarrhea. It was later reintroduced in 1958 by Ben Eiseman, a surgeon at the U. of Colorado who used fecal enemas to treat pseudomembranous colitis with an excellent response. Further interest in the microbiome has exploded in the last decade and the National Institutes of Health has launched the human microbiome project to catalog the microbial genes and species associated with the human body. The healthy gut microbiome primarily consists of ten species, with a predominance of Firmicutes and Bacteroidetes in healthy patients. Microbial colonization of the gut begins during birth and each individual develops their own specific gut microbiota.5 Gut dysbiosis is associated with many disease states including CDI, IBS (Irritable Bowel Syndrome), IBD (Inflammatory Bowel Disease), metabolic syndrome, obesity, multiple sclerosis, autism and diabetes.6,7 Among the factors that can disrupt the flora, antibiotics are the best understood; they affect both the size and composition of the microbiome, which can allow Clostridium difficile spores to dominate. FMT restores deficient Bacteroidetes and Firmicutes concentrations to healthy levels within two weeks.8
Although no definitive pathogen has been described for IBD, many believe a disturbance in the microbiome may play a part in its pathogenesis also,9 as decreased diversity of healthy species has been evident in the microbiome of IBD patients. The abnormal colonization may initiate excessive and unregulated inflammation that causes subsequent mucosal injury. Several studies have now reported that FMT may be a promising approach for management of IBD, though the results are mixed and not nearly as impressive or as reproducible as in RCDI.
TREATMENT OF CLOSTRIDIUM DIFFICILE
The first randomized controlled trial of FMT for RCDI enlisted 43 patients.10 The trial compared FMT via nasoduodenal tube after four to five days of oral vancomycin, vs. 14 days of vancomycin alone, vs. 14 days of vancomycin with bowel lavage. Resolution of symptoms occurred within three months in 81% of patients who received FMT, in 31 % who received vancomycin alone, and in 23% of patients who received vancomycin plus bowel lavage. The study was terminated early, since FMT was significantly more effective than vancomycin. A second randomized controlled study compared FMT delivered via nasogastric tube vs. colonoscopy.11 Symptoms resolved in 70% of patients after one FMT; the nasogastric group had a 60% cure, and colonoscopy had an 80% cure. The overall cure rate was 90% after retreatment.
A systematic review published in 2011 included 317 patients with RCDI treated with FMT.12 Symptoms resolved in 92% of patients; 89% after one treatment, and 5% more after retreatment. Another systematic review showed FMT successful in 85% of RCDI and 55% of refractory CDI, compared with medical success rates of 30%-80%.13 Presently, the evidence for using FMT for RCDI is more convincing than for fulminant or refractory CDI. Studies have also not yet supported FMT as an initial treatment for CDI. Future randomized controlled studies are needed in regards to using FMT as an initial treatment or for fulminant and refractory CDI.
Studies are also now examining different types of regimens for FMT. A recent study reported successful outcomes in two RCDI patients treated with a stool substitute of 33 different intestinal bacteria isolated in pure culture from a single donor, after having failed repeated courses of antibiotics.14 Another study showed that FMT with a frozen inoculum from a screened volunteer is as effective as fresh stool for treating RCDI.15 Finally, a feasibility study used frozen fecal capsules prepared from healthy donors to treat 20 patients with RCDI and found an overall response rate of 90% after one to two treatment courses.16
THE FMT PROCEDURE AT LGH
The use of FMT at LGH has advanced rapidly since the article about gut flora by Dr. Christopher Shih was submitted to this Journal in the fall of 2013, at which time FMT had not yet been performed here.17 In early 2013 the U.S. Food and Drug Administration (FDA) required an investigational new drug (IND) application for each FMT, which greatly restricted its implementation as routine therapy. This policy was changed, and the FDA now exercises discretion if patients are provided with an extended informed consent. Shortly after Dr. Shih’s article appeared, and in response to the FDA lifting requirements for an IND, this author submitted a protocol to LGH for FMT that — after considerable discussion and revision — was approved by the division. The first FMT in Lancaster was carried out at LGH in April 2014.
Current Indications
Clostridium difficile infection is defined according to the SHEA-IDSA guidelines: at least three unformed stools over 24 hours for two consecutive days and either positive stool testing (ELISA or PCR) for C. difficile toxins or pseudomembranes on colonoscopy.
FMT is used to treat CDI in the following circumstances:
1. Recurrent or relapsing CDI:
a. At least three episodes of mild-to-moderate CDI and failure of a six to eight-week taper with vancomycin, with or without an alternative antibiotic (e.g., rifaximin, nitazoxanide, fidaxomicin). (Relapsing CDI patients complete at least a 10-day course of vancomycin for the most recently diagnosed acute CDI prior to undergoing FMT.)
OR
b. At least two episodes of severe CDI resulting in hospitalization and associated with significant morbidity.
OR
2. Moderate CDI not responding to standard therapy (vancomycin) for at least a week. Refractory CDI patients have demonstrated no significant clinical improvement after at least seven days of higher dose vancomycin (250 mg QID).
Donor Eligibility Determination
We utilize a structured protocol for donor selection and screening. The patients may identify a donor such as a spouse /partner /intimate contact, a household family member [adult child, sibling], a first-degree family member outside the household [adult child, sibling], or another relative [aunt, uncle, cousin] or friend). Prospective donors undergo a brief medical interview as well as a screening questionnaire and laboratory testing.
Potential donors are evaluated to determine the presence of systemic medical conditions that would preclude donation (see following section also). These include a communicable disease, systemic autoimmune or atopic diseases, chronic pain syndromes (e.g. fibromyalgia, chronic fatigue), neurological disorders, malignancy, diarrheal disorders (IBS, IBD, celiac disease), use of antibiotics for any indication within the past three months, or obesity with a BMI >30.
We use a questionnaire based on the Donor History Questionnaire (DHQ) for screening blood donors. The DHQ is especially important to identify risks for diseases and conditions for which there are no laboratory tests, for which tests are not sensitive enough to detect infectious disease agents, and for which tests are unable to identify early stage or window-period infections.
Our modified DHQ must be completed within 30 days of FMT, and is used to exclude donors with risk factors such as high risk sexual behaviors (e.g. sexual contact with anyone with HIV/AIDS or hepatitis; men who have sex with men; those who have sex for drugs or money), known exposure to HIV or viral hepatitis within the previous 12 months, confinement in a correctional facility for more than 72 hours in the last 12 months, use of intravenous drugs or intranasal cocaine, recent tattooing or body piercing, recent transfusion, transplant or skin graft, risk factors for variant Creutzfeldt-Jakob disease.
Laboratory testing includes HIV 1 & 2 testing within two weeks of donation for FMT, as well as screening within one month for HAV IgM, HBsAg, anti-HBc (both IgG and IgM) and anti-HBs, HCV Ab, and RPR. Stool is tested for Clostridium difficile toxin by PCR; fecal Giardia or Cryptosporidium antigen; Ova and parasites; or Rotavirus via EIA. A bacterial culture checks for enteric pathogens (E coli, Salmonella, Shigella, Yersinia, Campylobacter) as well as Listeria monocytogenes and Vibrio (parahaemolyticus and cholerae); and an acid-fast stain is done for Cyclospora and Isospora.
Treatment Protocol
To prevent relapse while awaiting FMT, vancomycin is continued in relapsing subjects until two to three days prior to the scheduled procedure and by refractory subjects until the evening prior to the procedure. The day before the procedure, patients receiving endoscopic FMT are prepped with a standard bowel purge.
The procedure is performed in the GI endoscopy suite. The method used to deliver the FMT depends on the individual characteristics of the patient and is at the discretion of the treating physician. The methods used include colonoscopy, sigmoidoscopy, or enema.
Colonoscopy allows full endoscopic examination of the colon and exclusion of comorbid conditions (such as IBD, malignancy or microscopic colitis) which may have an impact on the patient’s treatment or response to therapy. This method has also yielded the highest cure rates. Sigmoidoscopy eliminates the additional risks associated with colonoscopy in patients who may not have a clear indication for colonoscopy, but still allows infusion of the stool into a more proximal segment of the colon than an enema. It does not require sedation, and may be beneficial in elderly or multiparous patients and may have difficulty retaining the material when given as an enema. Administration by enema allows infusion of the stool into the proximal segment of the colon and rectum without a sigmoidoscope. This method may be beneficial in patients who cannot be sedated and who are too unstable to be moved to the endoscopy suite. Delivery is via a Foley catheter, thus allowing the balloon of the Foley to obstruct the anus and assist in better stool retention. This method is preferred for fulminant critically ill patients due to the risks of endoscopic administration.
The physician administers 250-300 mL of the fecal suspension in aliquots of 60 mL.If done through a colonoscope or sigmoidoscope, it is delivered to the most proximal point of insertion. The patient is encouraged to retain stool for as long as possible (optimally two hours). After the procedure the patient is given one to two Loperamide tablets (optional at physician discretion) and is observed for two hours in the post- endoscopy unit for adverse reactions. Antibiotics are usually held after the FMT in most cases at the discretion of the physician.
Follow-up
We follow up with patients at seven and 30 days post-transplant. We tell patients to expect resolution of their symptoms within one to two weeks, and to call us with any sign of recurrent symptoms. Patients are also advised to avoid all antibiotics if possible for at least 30 days and as long as possible thereafter. The patient is given a list of “problem” antibiotics and asked to call our office if any antibiotic is started in the first 30 days as that causes a very high rate of relapse. Testing for Clostridium difficile is only performed if there are recurrent symptoms or if the stools remain loose and the patient requests repeat testing. Solid stools are not retested for Clostridium difficile.
Patients who fail a first FMT are offered a second FMT and are usually treated with Vancomycin or Dificid for 10-14 days (see below for our cure rates with second FMTs).
Adverse Events
There are three areas of risk associated with treatment: 1) Physical risks related to the colonoscopy or sigmoidoscopy (i.e. perforation, abdominal pain, bleeding, anesthesia reaction, etc.); 2) Theoretical risks (infectious and otherwise) related to FMT (extremely rare due to rigid screening of donors); and 3) Psychological or other risks related to confidentiality and loss of privacy.
There have been reports of Norovirus transmission,18 Bacteremia in IBD,19 and flares of IBD with FMT,20 but these have been quite rare. One study followed 77 patients after colonoscopic FMT21 and found that after three months to several years 91% of patients achieved primary cure and 98% were secondarily cured (after additional FMT, antibiotics, or probiotics). In that study, three patients developed new immune conditions but it is not known if they were directly related to FMT. The LGH results are discussed below.
Results of FMT at Lancaster General Health
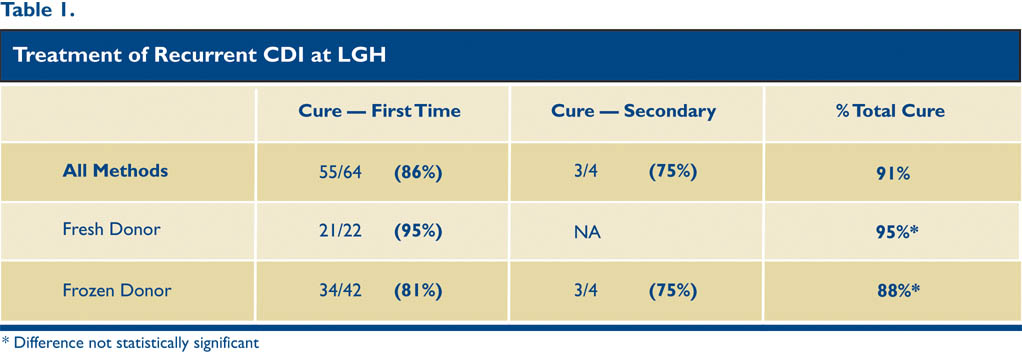
Our group has now performed 64 FMTs for RCDI with primary cure in 55 of those patients (86%), comparable to the literature. Of the nine relapses, most occurred within the first 30 days, and thus far four of those patients have been re-transplanted with cure of three of the four patients, making our overall cure rate 91%. (See Table 1).
Frozen Stool
Frozen stool was approved for use at LGH in September 2014 and is now the predominant method used. Twenty-two of our 64 patients were transplanted with fresh stool while the remaining 42 received frozen stool. All of the frozen samples are currently obtained from Open Biome stool bank in Massachusetts, a nonprofit stool bank started by two MIT students. It has provided over 6,000 stools for FMT and uses high quality screening methods. All of the pertinent screening lab work is sent with the corresponding stool samples and scanned into the electronic patient record at LGH. This stool bank has allowed many patients to be treated more efficiently, cost effectively, and safely, and many patients are now choosing this option. Five frozen stools from donor labs are stored in the LGH endoscopy department at all times.
With FMT for fulminant or refractory cases in critically ill hospitalized patients our cure rates are lower — 40%-50% on a much smaller patient population — and the adverse event rate is higher. Results like these are common in the literature, and multiple studies are underway to determine what modifications to current FMT protocols will help improve cure rates in this difficult population of patients.
As to complications, we have had one colonic perforation, although this was likely related to a polypectomy performed at the time of FMT. We have also had three aspirations during FMT although these complications all occurred on patients with fulminant disease. Otherwise, the complications have been: mild abdominal pain for two to three days, and malaise for one to two days.
FUTURE DIRECTIONS FOR FMT
Ulcerative Colitis
The first case report of FMT for UC was published in the Lancet in 1989.22 The authors themselves had UC refractory to steroids and treated it with FMT consisting of large volume retention enemas from healthy donors. Colonoscopic biopsies at three months showed resolution of inflammation, and at six months the authors were symptom free. Many other small studies showed mixed results using FMT for Ulcerative Colitis. In 2013 a prospective study used FMT in five patients with moderate to severe ulcerative colitis.23 No patients achieved remission and only one patient had a symptom response. Two randomized controlled studies using FMT for UC were published recently and both were stopped early for futility.24,25 Follow up results of one of these studies showed remission in 24% compared to controls at 5%. Larger studies are needed and researchers need to examine if methods should be altered for FMT in UC vs. the methods used for RCDI.
Crohn’s disease
The data for treating Crohn’s disease with FMT are sparser and are limited to small studies. A study from China reported results of a single FMT delivered mid-gut in 30 patients with refractory Crohn’s disease.26 Although follow up was only for one month, rates of improvement and clinical remission were 86% and 76% respectively. A 2014 case report showed remission in a patient that had failed prior immunosuppressive therapy.27 The study analyzed the patient’s stool pre and post FMT and the change in microbiome did not persist as it does for RCDI. This finding suggests that repeated transplants of FMT, or perhaps pill delivery systems, may be more beneficial in Crohn’s disease.
A series of randomized trials is currently underway of FMT in the treatment of Crohn’s disease. Many of these are listed at clincaltrials.gov.
Functional GI disorders
In contrast to the more serious IBD (Irritable Bowel Disease), early results in studies using FMT for IBS (Irritable Bowel Syndrome) have been more positive. A recent study showed that IBS patients have a significant decrease in the numbers of Bacteroidetes and Faecalibacterium species and a significant increase in Firmicutes.28 This alteration may uncover a link between the brain- gut axis in IBS and suggests that FMT may be beneficial.
One study examined 13 patients with both constipation and diarrhea — predominant IBS and their response to FMT.29 Seventy percent reported resolution or improvement in multiple symptoms of IBS especially abdominal pain, bowel habits, and bloating. In another series of 45 patients with chronic constipation treated by FMT, 89% had symptomatic relief and 18 reported normal bowel habits during follow up of 9-19 months.30 Another pilot study examined FMT in 24 patients with slow transit constipation.31 The rates of clinical improvement and remission were 50% and 37% respectively, accompanied by a decrease in colonic transit time after FMT.
The data for the use of FMT to treat IBS are promising, but larger randomized controlled studies are needed to further explore these results.
Diabetes and Obesity
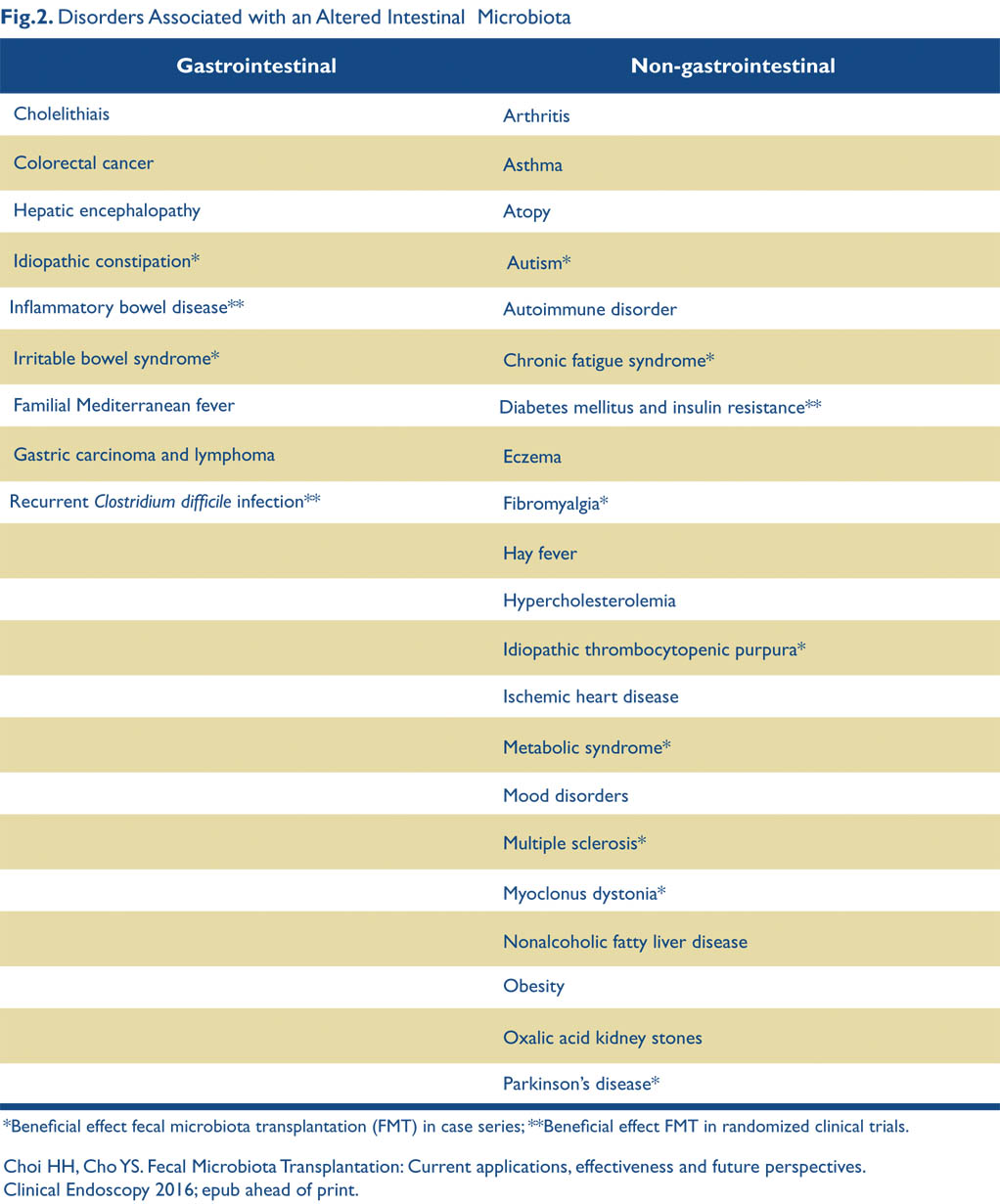
A double-blind, randomized, controlled study examined the use of FMT for diabetes and obesity in 18 male subjects.32 The study demonstrated improved fasting triglycerides and insulin resistance. Other smaller studies have shown similar results but no study has yet shown a decrease in weight using FMT. Obviously, this is another exciting area of potential treatment with FMT. Fig. 2 lists gastrointestinal and other disorders being studied as potential treatment targets for FMT, and illustrates that interest in using the microbiome as a treatment target is quickly expanding.
CONCLUSIONS
FMT has demonstrated impressive efficacy in the treatment of recurrent Clostridium difficile infections, with cure rates of 80%-95% in many well performed studies. Success in this disease state has led to an explosion of research in the microbiome and its potential in the treatment of other GI and non GI diseases.
Many questions remain with FMT despite its high success rate with RCDI. Why does it work? Is it specific bacteria or groups of bacteria? Is there a “super donor?” What is the role of bacteria on inflammation and autoimmunity? Do different approaches or repeated applications need to be done in IBD, IBS and other disease states for efficacy? Will pill formulations be a much more attractive and reproducible delivery mechanism in the near future, especially in IBD and IBS?
The early results remain promising but many more randomized, controlled studies are needed to standardize all aspects of FMT and delineate other areas with potential for treatment success. We are encouraged with our local success with FMT for RCDI and remain committed to utilizing the microbiome and its potential for treatment of many disease states in the future.
REFERENCES
1. Kelly C. A 76 Year Old Man with Recurrent Clostridium difficile Associated Diarrhea: Review of C difficile Infection. JAMA 2009; 301(9):954-62.
2. Johnson S, Schriever C, Galang M, et al. Interruption of recurrent Clostridium difficile associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis. 2007; 44(6):846-48.
3. Wilcox MH. Descriptive Study of intravenous immunoglobulin for the treatment of recurrent Clostridium difficile diarrhea. J Antimicrob Chemother. 2004; 53(5):882-84.
4. Zhang F, Luo W, Shi Y, Fan Z, Ji G. Should we standardize the 1,700 year old fecal microbiota transplantation? Am J Gastroenterol 2012; 107:1079-1087.
5. Shih, C. Gut Flora. J Lanc Gen Hosp. 2013; 8: 114-117.
6. Sommer F, Backhed F. The gut microbiota:masters of host development and physiology. Nat Reve Microbiol 2013:11:227-238.
7. Britton RA, Young VB. Role of the intestinal microbiota in resistance to colonization by Clostridium difficile. Gastroenterology 2014; 146: 1547-1553.
8. Khoruts A, Dicksved J, Jansson JK, Sadowsky MJ. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile associated diarrhea. J Clin Gastoenterol 201; 44: 354-60.
9. Cammarota G, Ianrio G, Cianci R, Bibbo S, Gasbarini A, Curro D. The involvement of gut microbiota in inflammatory bowel disease pathogenesis: potential for therapy. Pharmacol Ther 2015; 149: 191-212.
10. van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium Difficile. N Engl J Med 2013; 368: 407-15.
11. Youngster I, Sauk J, Pindar C, et al. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum of unrelated donors: a randomized, open-label, controlled pilot study. Clin Infect Dis 2014;58: 1515-22.
12. Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection 2011; 53: 994-1002.
13. Drekonja D, Reich J, Gezhegn S, et al. Fecal microbiota transplantation for clostridium difficile infection: a systematic review. Ann Intern Med 2015; 162: 630-38.
14. Patel NC, Griesbach CL, DiBiase JK, Orenstein R. Fecal microbiota transplant for recurrent Clostridium difficile infection. Mayo Clinic in Arizona experience. Mayo Clin Proc 2013; 88: 799-805.
15. Lee Ch, Steiner T, Petrof EO, et al. Frozen vs. fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection: A randomized controlled trial. JAMA 2016; 315 (2); 142-9.
16. Youngster J, Russell GH, Pindar C, Ziv-Baran T, Sauk J, Hohmann EL. Oral capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA 2014; 213:1772-78.
17. Shih C. The Gut Flora. J Lanc Gen Hosp. 2013; 8: 114-117
18. Schwartz M, Gluck M, Koon S. Norovirus gastroenteritis after fecal microbiota transplantation for treatment of Clostridium difficile infection despite asymptomatic donors and lack of sick contacts. Am J Gastroenterol. 2013 Aug; 108(8):1367.
19. Quera R, Espinoza R, Estay C, Rivera D. Bacteremia as an adverse event of fecal microbiota transplantation in a patient with Crohn's disease and recurrent Clostridium difficile infection. J Crohns Colitis. 2014 Mar;8(3):252-3.
20. De Leon LM, Watson JB, Kelly CR. Transient flare of ulcerative colitis after fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin Gastroenterol Hepatol. 2013 Aug; 11(8):1036-8.
21. Brandt LJ, Aroniadis OC, Mellow M, et al. Long-term follow up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroentrol 2012; 107: 1079-87.
22. Bennett JD, Brinkman M. Treatment of ulcerative colitis by implantation of normal colonic flora. Lancet 1989; 1: 164.
23. Angelberger S, Reinisch, W, Makristathis A, et al. Temporal bacterial community dynamics vary among ulcerative colitis patients after fecal microbiota transplantation. Am J Gastroenterol 2013; 37:42-47.
24. Moayyedi P, Surette M, Kim P, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 2015; 149:102-109.
25. Rossen N, Fuente S, van der Spek M, et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology 2015; 149: 110-18.
26. Cui B, Feng Q, Wang H, et al. Fecal microbiota transplantation through mid-gut for refractory Crohn’s disease: safety, feasibility, and efficacy trial results. J Gastroenterol Hepatol 2015; 30:51-58.
27. Kao D, Hotte N, Gillevet P, Madsen K. Fecal microbiota transplantation inducing remission in Crohn’s colitis and the associated changes in fecal microbial profiles. J Clin Gastroenterol 2014; 48:625-28.
28. Rajilic-Stojanovic M, Biagi E, Heilig HG, et al. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology 2011; 141: 1792-1801.
29. Pinn DM, Aroniadis OC, Brandt LJ. Is fecal microbiota transplantation the answer for irritable bowel syndrome? A single center experience. Am J Gastroenterol 2014; 109:1831-32.
30. Andrews P, Barody TJ, Shortis NP, Thompson S. Bacteriotherapy for chronic constipation: a long term follow-up. Gastroenterology 1995; 108(4 suppl 2):A 563.
31. Tian H, Ding C, Gong J, et al. Treatment of slow transit constipation with fecal microbiota transplantation: A pilot study. J Clin Gastroenterol 2016; epub ahead of print.
32. Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012; 143: 913-16.