
Fall 2016 - Vol. 11, No. 3
Antibiotic Stewardship in Ambulatory Care Settings
and Surveillance for Enteric Pathogens in Pennsylvania
Nkuchia M. M'ikanatha, DrPH, MPH
Lead Epidemiologist for Antimicrobial Resistance Response
Pennsylvania Department of Health
ABSTRACT
The world is facing an imminent crisis in the control of infectious disease as a result of the gradual but steady increase in the resistance of many pathogens to available therapeutic drugs.
1,2 Moreover, use of antibiotics of last resort, such as carbapenems, is increasing. Use of last resort drugs raises special concerns because of their potential to exacerbate selection of fully resistant bacterial populations, thus undermining their role in treatment of serious infections. In the United States each year, an estimated 2 million antibiotic resistant infections occur resulting in 23,000 deaths and huge economic costs. Overuse and misuse of antibiotics contributes to the emergence of resistance. A significant proportion of the approximately 10 million antibiotics prescribed annually to patients for upper respiratory tract infections (UTI) are unnecessary because most UTIs are caused by viruses. Pennsylvania has implemented Get Smart initiatives to promote antimicrobial stewardship, and an integrated surveillance program to monitor emergence of resistance in enteric bacteria.
The recent national plan to combat antibiotic-resistant bacteria, combined with strong leadership at the state level, has strengthened public health responses. However, partnerships among key stakeholders, including physicians, patients, medical practices, and professional organizations, are fundamental to the success of public health responses.
OVERVIEW
Increasing antimicrobial resistance makes it difficult to predict which antibiotics will be effective for management of serious infections. It is no longer rare for a microbiology report to show very limited choices for effective antibiotics. Alexander Fleming’s words regarding antibiotic resistance were prophetic; in his Nobel Prize lecture on December 11, 1945, he noted that microbial resistance to the newly discovered penicillin already occurred both in the laboratory and in the human body.
3
Most clinicians and epidemiologists realize that reports about nightmare drug-resistant bacteria no longer belong exclusively in supermarket tabloids but are a real threat to the practice of medicine in the 21st century. This article offers a perspective on factors driving emergence of resistance and highlights core recommended actions. It also offers an example of antimicrobial stewardship in ambulatory care settings in Pennsylvania, and briefly discusses surveillance efforts.
The World Health Organization (WHO) recently characterized pathogen resistance to advanced cephalosporins, including resistance conferred by extended spectrum beta-lactamases (ESBLs), as a global threat. In a typical scenario, the use of carbapenems to treat severe infections caused by ESBL–producing E. coli leads to emergence of resistance to these drugs, further limiting choices for treatment.
4
The statistics are sobering. The Centers for Disease Control and Prevention (CDC) estimates that each year at least 2 million people acquire serious infections due to bacteria that are resistant to one or more therapeutic agents, resulting in 23,000 deaths each year.
5 Conservatively, these infections result in $20 billion in direct health care costs with an additional $35 billion attributed to lost productivity.
6 National active surveillance efforts recently estimated the yearly burden of Clostridium difficile infections (CDI) at 453,000 incident infections, resulting in 29,000 deaths.
7 Although other factors, including advanced age, are associated with CDI, prior antibiotic use is the single most important risk factor. Antibiotics can alter intestinal microbiota for three months or more, which increases susceptibility to infections.
8,9 Additionally, antibiotics are associated with over 140,000 drug-related emergency department visits each year, most of which are caused by allergic reactions that range from mild rashes to breathing difficulties.
10
Antibiotics are often prescribed in outpatient settings for self-limiting acute illnesses, particularly upper respiratory tract infections (URTIs), even though it is widely recognized that most of these infections are viral and the majority of antibiotic prescriptions for URTIs are unnecessary. Furthermore, an estimated 50 million courses of antibiotics are prescribed annually for children in ambulatory settings. Since about 10 million are for URTIs,
11 a substantial proportion of this antibiotic use is unnecessary. In the United States, approximately 842 antibiotic prescriptions per 1,000 persons are written annually, with striking regional variations ranging from 931 per 1,000 persons in the South, to 647 per 1,000 in the West.
12 Here in Pennsylvania, our rate of approximately 804 prescriptions per 1,000 persons annually is consistent with the national average.
Clearly, both patients and providers should carefully weigh the risks versus benefits of antibiotics. However,
minimizing the societal consequences of widespread antibiotics use requires concerted efforts that transcend individual patients and their providers.
PUBLIC HEALTH RESPONSES TO ANTIMICROBIAL RESISTANCE
The 2015 National Action Plan for Combating Antibiotic-Resistant Bacteria offers a clear path for prevention of a nightmare scenario where health care providers have little or no treatment option for patients with bacterial infections.
13 This timely document offers practical steps to achieve recommendations made in September 2014 by the President’s Council of Advisors on Science and Technology (PCAST) in response to President Barack Obama’s Executive Order 13676: Combating Antimicrobial-Resistance Bacteria.
14
The National Action Plan’s goals are:
1. To slow emergence of resistant bacteria and prevent the spread of resistant infections through implementation of public health programs and reporting policies that advance antibiotic-resistance prevention and foster antibiotic stewardship in health care settings and the community.
2. To strengthen national One-Health surveillance efforts to combat resistance through multiple objectives including antibiotic susceptibility testing and molecular characterization of select zoonotic and animal pathogens.
GET SMART: KNOW WHEN ANTIBIOTICS WORK
To promote antimicrobial stewardship, the Pennsylvania Department of Health has implemented the Get Smart: Know When Antibiotics Work Program. This program is focused on decreasing inappropriate use of antibiotics for common illnesses in ambulatory care settings. A related goal is to reinforce primary preventive measures including hand hygiene, infection control, and recommended vaccinations.
Studies have demonstrated that in ambulatory care settings, the expectations, knowledge, and prior experience of patients or – in the case of children – their parents, influence whether or not a physician writes an antibiotic prescription for a URTI.
15,16 With this in mind, the Get Smart Program draws from resources in academic institutions, health care organizations, health plans, and others, in addition to government resources at the state and federal level. Collaborative efforts are grouped into five initiatives based on setting, target population, and types of activities (see Table 1).
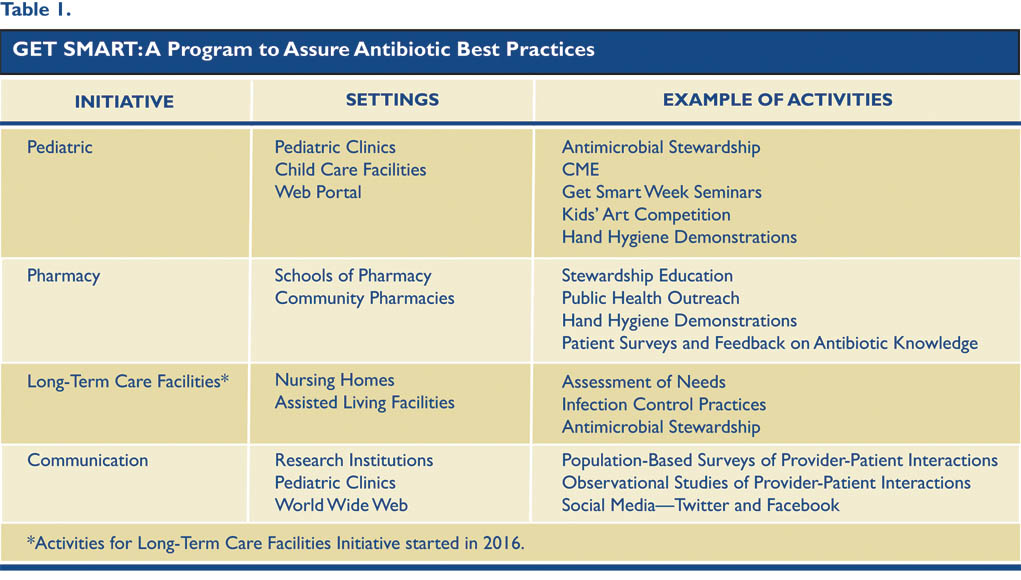
Projects and activities have been implemented under each initiative to meet the following key objectives:
• Promote guidelines for antimicrobial stewardship
• Decrease consumer demand for unnecessary antibiotics
• Increase adherence to preventive measures—infection control, personal hygiene, and recommended vaccinations.
The key objectives of Pennsylvania’s program are derived from national goals developed by CDC’s Get Smart: Know When Antibiotics Work Program. Activities are implemented based on known best practices and lessons learned by federal and similar programs in other jurisdictions. Evidence from multiple data sources has demonstrated that targeted educational efforts to promote judicious use of antibiotics make an impact. Data from CDC show a 24% cumulative reduction in antibiotic use between 1993 and 2008 in children younger than 14 years – from 300 antibiotic courses per 1,000 office visits in 1993-94 to 229 courses in 2007-08.17 For children less than 5 years of age, an impressive decrease in antibiotic prescriptions of 36% occurred from 1995 to 2006 (from 1,216 to 779 per 1,000 population), primarily because there were fewer office visits by children in this age group for otitis media and fewer prescriptions for acute respiratory tract infections.18
To complement the state response to the escalating threat of antimicrobial resistance, the Pennsylvania Consortium for Antimicrobial Stewardship (PCAS) was recently created. PCAS fosters collaborations among individuals in diverse disciplines including physicians, biomedical and behavioral scientists, epidemiologists, and veterinarians. The consortium supports hosting of Get Smart Week seminars, exhibits at public health and large public forums, and conducts focused research to support other initiatives. Participating institutions include Penn State College of Medicine, Penn State Department of Communication Arts and Sciences, and the University of Pennsylvania and the University of Pittsburgh Schools of Pharmacy and Medicine.
PEDIATRIC INITIATIVE
Although there had been earlier efforts to promote judicious use of antibiotics, implementation of the current initiatives started in 2005 as a collaboration among the Penn State College of Medicine, the University of Pennsylvania Center for Clinical Epidemiology and Biostatistics, and the Department of Human Services. With support from the CDC Get Smart Program, the Pennsylvania Department of Health implemented a pediatric initiative in 2005, primarily focused on increasing awareness about appropriate antibiotic use in child care settings.
To capture current practices, in 2007 a survey was conducted of Directors of randomly selected Pennsylvania child care facilities to assess whether policies that excluded sick children played a role in overuse of antibiotics. Survey authors hypothesized that the requirement that children be evaluated and treated before returning to the child care facility put pressures on parents to seek antibiotics for their children. Fifty-two percent of the 135 respondents agreed that children are prescribed antibiotics unnecessarily, and 89% believed that parents pressure physicians to prescribe unnecessary antibiotics.19 Taken together with data from other studies, insights from this study informed design of our current pediatric initiative, which focuses on educating child care providers about current guidelines for management of common childhood illnesses, including preventive measures such as vaccinations and hand hygiene practices. As part of the pediatric initiative, an advisory group that included pediatricians, child care directors, and public health and policy officials produced the report Practical Consideration in Implementation of ModelSick Exclusion Policy in Childcare Settings, which recommends exclusion criteria.
COMMUNICATION INITIATIVE
The Communication Initiative supports other activities through dissemination of training materials, organization of large events, and an annual art competition open to children. Additionally, the team is conducting behavioral research to identify the factors that drive overuse of antibiotics. The first major project under this initiative began in 2012 with release of a Get Smart web portal (knowwhentosayno.org) developed in collaboration with the Perelman School of Medicine. This site provides easily accessible practical information to assist in prevention of common childhood illnesses in child care settings. In addition, this site has resources on antimicrobial stewardship including copies of recent continuing medical education (CME) presentations.
To create awareness about the website, the Pennsylvania Get Smart Program began what is now an annual art competition in 2013. Typically, the Physician General announces the competition by a press release to media in mid-March and it concludes the end of October. The Physician General announces winners during the national annual Get Smart About Antibiotics Week in the third week of November. All these announcements are immediately posted on the website and recent data suggest that the strategy increases awareness. Three hundred eighty-eight children from 31% of counties in Pennsylvania (21 of 67) submitted entries in 2013. Based on subsequent evaluations, the drawing competition engaged not only children but also their parents and child care providers. This suggests that a simple innovation like this could be used to promote engagement.20
To raise awareness, antimicrobial stewardship forums are held in institutional settings including child care facilities, health care institutions, and college campuses. During an antimicrobial stewardship event on November 17, 2015, at University Park, the Pennsylvania Physician General Rachel Levine, M.D., read a proclamation declaring November 16-22, 2015, Get Smart About Antibiotics week in the Commonwealth. A CME presentation reviewed outpatient antimicrobial stewardship and examples of core strategies: prior authorization, prospective audit and feedback, and formulary restriction. It also provided clinical guidelines for antibiotic dose route (recommending changes from intravenous to oral), dose optimization, and antimicrobial order forms.21 Lively discussion among the audience members suggested that clinicians do not perceive that their own prescribing contributes to antibiotic overuse or that they acquiesce to parental pressure to prescribe antibiotics. In fact, studies suggest that clinicians who succumb to pressure fear losing patients to other practices who would “give them what they want.”22 Physicians with Penn State student health service shared anecdotal evidence of parental pressure including pressure by influential donors to the University.
INTEGRATED SURVEILLANCE FOR ENTERIC PATHOGENS
As part of the mandated reporting of specific diseases and conditions by clinical laboratories, physicians, and others specified by regulations, the Pennsylvania Department of Health integrated surveillance for antimicrobial resistance in enteric bacterial pathogens (e.g., Salmonella and Campylobacter).23 Mandated reporting is the first step in surveillance for, and control of, infectious diseases. Health care providers have a crucial role in the success of the program by diagnosing infections designated to be of public health importance, and reporting them (usually via laboratories) to public health authorities.
Monitoring antimicrobial resistance in clinical enteric bacterial isolates
Since 2003, Pennsylvania has participated in submission of selected enteric isolates associated with sporadic illnesses and outbreaks to the National Antimicrobial Resistance Monitoring System (NARMS) laboratory at the CDC. Of the isolates received at the state lab, every twentieth non-typhoidal Salmonella, Shigella, and Escherichia coli O157 isolate and every Salmonella serotype Typhi, serotype Paratyphi A, serotype Paratyphi C, and Vibrio (other than V. cholerae) isolate to the CDC. The CDC NARMS lab tests these isolates, along with those received from other states, for susceptibility to antimicrobial agents that are clinically important for treatment of the specific pathogen. For example, Salmonella isolates are tested for susceptibility to fluoroquinolones (e.g., ciprofloxacin) and third-generation cephalosporins (e.g., ceftriaxone), commonly used to treat severe infections. In addition to antibiotic resistance data received from the CDC NARMS, the Pennsylvania Department of Health conducts integrated surveillance to better characterize selected bacterial isolates using antimicrobial susceptibility testing and molecular subtyping by pulsed-field gel electrophoresis (PFGE).24 In the future, whole genome sequencing is expected to replace PFGE to enhance outbreak detection and elucidate resistance mechanisms.
Monitoring antimicrobial resistance in enteric bacterial isolates from retail meat
Changes in antimicrobial susceptibility in bacterial isolates from food have been tracked since 2006 as a collaborative pilot study partly supported by the Center for Clinical Epidemiology and Biostatics at the University of Pennsylvania School of Medicine. The pilot study tested raw chicken meat samples purchased from randomly selected retail outlets in central Pennsylvania during 2006–2007 for the presence of Salmonella. Of 378 chicken meat samples, 84 (22%) contained Salmonella, and 26 (31%) of the Salmonella isolates were resistant to three or more antimicrobials. Eighteen of the multi-drug resistant Salmonella isolates had a gene known to confer resistance to both ceftiofur, used in poultry, and ceftriaxone, used in humans. One of the isolates that carried this gene exhibited a rare PFGE pattern indistinguishable from an isolate recovered in a child with salmonellosis. (Fig. 1).

Fig. 1. One antibiotic-resistant salmonella serovar typhimurium chicken isolate was indistinguishable from an antibiotic-resistant isolate from an ill 17-year-old Philadelphia resident. Two-enzyme PFGE analysis was used to achieve high discriminatory power.
This study demonstrated that molecular subtyping can be used in epidemiological investigations to associate human illnesses, and it suggested that chicken was a source of multidrug-resistant Salmonella.25 After the pilot study, Pennsylvania joined ten other states to conduct retail meat surveillance in an effort coordinated by the FDA. Today fourteen states are part of this FDA-supported program, and all states contribute clinical isolates to the CDC (Fig. 2).
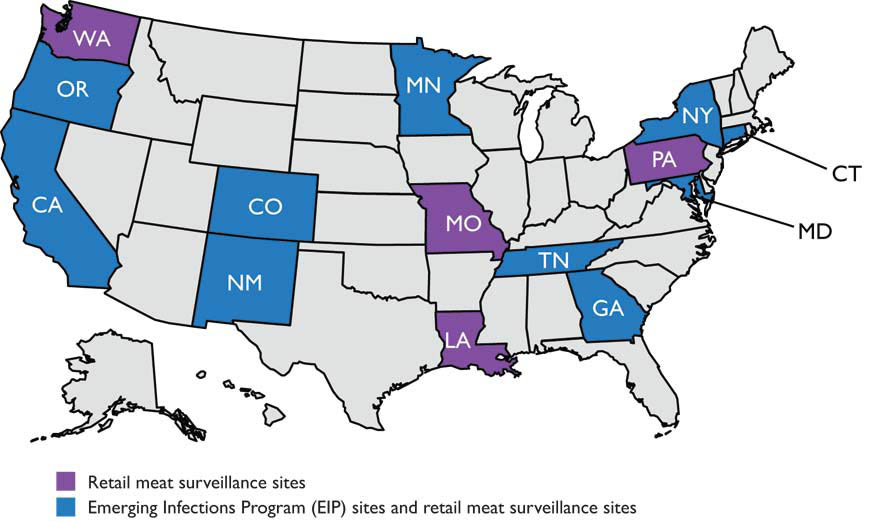
Fig. 2. As of July 2016, 14 retail meat surveillance sites, including Pennsylvania, tested for enteric bacteria from retail meat samples (chicken parts, ground turkey, pork chops and ground beef). All sites culture for salmonella (all meat types) and campylobacter (poultry). Sites send isolates to the FDA for confirmation, serotype/speciation, susceptibility testing, and additional testing.
SUCCESS, CHALLENGES, OPPORTUNITIES AND FUTURE DIRECTIONS
Recently, initiatives led by officials at both the national and state levels have invigorated public health efforts to combat antimicrobial resistance. Here in Pennsylvania, Physician General Rachel Levine’s efforts resulted in the governor’s proclamation for observance of Get Smart Week in Pennsylvania and events throughout the year to promote judicious antibiotic use.
Unfortunately, recent evidence suggests that the reduction in antibiotic prescriptions for children has plateaued and that there has been an increase in broad-spectrum antibiotic use for particular conditions.17 An example is an increase in use of third-generation cephalosporins for treatment of otitis media.18 Furthermore, antimicrobial resistance genes are now ubiquitous in a highly dynamic environment and can be newly acquired by pathogenic bacteria. The threats of antimicrobial resistance are complex and rapidly evolving.2
The expansion of initiatives to combat drug-resistant pathogens will require substantial resources. Furthermore, antibiotic stewardship programs run within hospitals and ambulatory clinics are of paramount importance and warrant strong local administrative support. Long-term care centers are increasingly recognized as potentially high-risk settings for transmission of multi-drug resistant bacteria—this population has generally been underserved in terms of antibiotic stewardship programs. For these reasons, strong local partnerships among key stakeholders, including academic medical centers, group and individual medical practices, professional organizations, and individuals, are fundamental to the success of public health responses to the threat of antimicrobial resistance. These efforts will ensure our coexistence with formidable microbes in a perpetual challenge succinctly described by Dr. Joshua Lederberg, Nobel Laureate, as “episodes of our wits versus their genes.”26
REFERENCES
1. Smolinski MS, Hamburg, M.A., Lederberg J (eds.), eds. Microbial threats to health: emergence, detection, and response. Washington, D.C, National Academies Press, 2003.
2. Kontra J, The Perfect Storm: Worsening Microbial Resistance and Too Few New Antibiotics? J Lanc Gen Hosp. 2013; 8(3): 73-76. http://www.jlgh.org/Past-Issues/Volume-8---Issue-3/The-Perfect-Storm--Worsening-Microbial-Resistance.aspx
3. Fleming A. Penicillium: Nobel Lecture. http://www.nobelprize.org/nobel_prizes/medicine/laureates/1945/fleming-lecture.pdf
4. World Health Organization. Antimicrobial resistance: global report on surveillance 2014. World Health Organization (2014). Geneva, Switzerland. http://www.who.int/drugresistance/documents/surveillancereport/en/.
5. CDC. Antibiotic Resistance Threats in the United States, 2013. Atlanta, GA:U.S. Centers for Disease Control and Prevention (2013). Accessed May 5, 2016 at http://www.cdc.gov/drugresistance/threat-report-2013/
6. The Alliance for the Prudent Use of Antibiotics (APUA). The cost of antibiotic resistance to U.S. families and the health care system. Accessed May 5, 2016 at: http://www.tufts.edu/med/apua/consumers/personal_home_5_1451036133.pdf
7. Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015;372:825-834
8. Henrich TJ, Krakower D, Bitton A, et al. Clinical risk factors for severe Clostridium difficile-associated disease. Emerg Infect Dis 2009; 15:415–22
9. Shih C. The Gut Flora. J Lanc Gen Hosp. 2013; 8 (4): 114-117. http://www.jlgh.org/Past-Issues/Volume-8---Issue-4/The-Gut-Flora.aspx
10. Shehab N, Patel PR, Srinivasan A, et al. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008; 47:735-43. doi: 10.1086/591126.
11. Hersh AL, Shapiro DJ, Pavia AT, et al. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics. 2011;128(:1053–1061pmid:22065263.
12. Hicks LA, Bartoces MG, Roberts RM, Suda KJ, Hunkler RJ, Taylor TH Jr, Schrag SJ. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011.Clin Infect Dis. 2015 ;60:1308-16.
13. The White House. (2015). National Action Plan for Combating Antibiotic-Resistant Bacteria. Accessed April 22, 2016 at https://www.whitehouse.gov/sites/default/docs/national_action_plan_for_combating_antibiotic-resistant_bacteria.pdf
14. President’s Council of Advisors on Science and Technology. (2014). Report to the President on Combating Antibiotic Resistance. Presidential Executive Office of the President. Accessed April 22, 2016 at:. http://www.whitehouse.gov/sites/default/files/microsites/ostp/PCAST/pcast_carb_report_sept2014.pdf
15. MacFarlane J, Holmes W, MacFarlane R, Britten N. Influence of patients’ expectations on antibiotic management of acute lower respiratory tract illness in general practice: questionnaire study. BMJ. 1997;315:1211–1214
16. Huang SS, Rifas-Shiman SL, Kleinman K, et al. Parental knowledge about antibiotic use: results of a cluster-randomized, multicommunity intervention. Pediatrics. 2007;119:698-706.
17. McCaig LF, Hicks L, Roberts R, Fairlie T, Centers for Disease Control and Prevention (CDC) Office-related antibiotic prescribing for persons aged ≤ 14 years—United States, 1993-1994 to 2007-2008. MMWR Morb Mortal Wkly Rep. 2011;60(34):1153–1156pmid
18. Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302(7):758–766pmid
19. M'ikanatha NM, Gaskin LB, Kunselman A, Warren K, Lautenbach E. Child care center exclusion policies and directors' opinions on the use of antibiotics. Infect Control Hops Epidemiology. 2010;31: 408-11
20. M’ikanatha NM, Mueller N, Boktor Sw. Use of a Drawing Competition to Create Awareness about an Antimicrobial Stewardship Website. Council of State and Territorial Epidemiologist Annual Meeting. Boston, MA, 6/14-6/18,2015. Accessed on April 16, 2016 at: https://cste.confex.com/cste/2015/webprogram/Paper5108.html
21. Gerber SJ. Get Smart Week Presentation: Outpatient Antimicrobial Stewardship. Accessed on April 23, 2016 at: http://www.med.upenn.edu/antibiotics/presentations/2015_ABX%20STW%20Methods_Gerber_Nov17_2015.pdf
22. Dempsey PP, Businger AC, Whaley LE, et al. Primary care clinicians' perceptions about antibiotic prescribing for acute bronchitis: a qualitative study. BMC Fam Pract. 2014;15:194.
23. Pennsylvania Department of Health. List of Reportable Diseases. Accessed on April 9,2016 at: http://www.health.pa.gov/Your-Department-of-Health/Offices%20and%20Bureaus/epidemiology/Pages/Reportable-Diseases.aspx#.VwlAYeApCpo.
24. M’ikanatha NM, Sandt HS, Chen SW, Dettinger LA, Perry B, Achenbach J, Li YL, Ostroff S. An integrated approach to improve surveillance for foodborne pathogens of animal origin. IDWeek 2012, San Diego, CA. Accessed on April 29,2016 at: https://idsa.confex.com/idsa/2012/webprogram/Paper36596.html
25. M'ikanatha NM, Sandt CH, Localio AR, Tewari D, Rankin SC, Whichard JM, Altekruse SF, Lautenbach E, Folster JP, Russo A, Chiller TM, Reynolds SM, McDermott PF. Multidrug-resistant Salmonella isolates from retail chicken meat compared with human clinical isolates. Foodborne Pathog Dis. 2010;7:929-34.
26. Lederberg J. Infectious history. Science 2000; 288:287–93.