
Spring 2016 - Vol. 11, No. 1
An Introduction to Heart Failure
in Children
Matthew J. O'Connor, M.D.
Assistant Professor of Clinical Pediatrics, Division of Cardiology
The Children's Hospital of Philadelphia
University of Pennsylvania Perelman School of Medicine
INTRODUCTION
As defined by the American Heart Association,heart failure (HF) is a “complex clinical syndrome that results from any structural or functional impairment of ventricular filling or ejection of blood.”
1 Encompassed within this definition are HF with reduced ejection fraction (systolic ventricular dysfunction) or preserved ejection fraction (diastolic ventricular dysfunction). Although HF in adults is common and has considerable impact on health care outcomes, in children it is relatively rare, and may not be seen often in community practice settings. Nonetheless, prompt recognition of HF in children is important, as prompt referral may limit unnecessary investigations and even be lifesaving for critically ill children. This review will define the clinical syndrome of HF in children and examine its etiology, symptomatology, evaluation, and treatment. Given the rarity of HF with preserved ejection fraction in children, unless described otherwise the term HF in this review refers to HF with reduced ejection fraction, and the focus will be on HF caused by left ventricular systolic dysfunction.
ETIOLOGY OF HEART FAILURE IN CHILDREN
The etiologies leading to HF in children and adults overlap, but their most common etiologies differ widely. While there is no ideal classification scheme, the etiology of HF in children can best be approached by first considering the presence or absence of structural heart disease.
A proposed list of HF etiologies in children is presented in Table 1.

For the purposes of this article, “structural heart disease” encompasses primary anatomic cardiac abnormalities such as congenital heart disease (CHD), valvular stenosis/regurgitation independent of a congenital abnormality, and coronary artery anomalies. (These etiologies are distinct from cardiomyopathies and pericardial disease.) In children, CHD is clearly an important etiology, whereas acquired heart disease, namely coronary artery disease, predominates in adults.
CONGENITAL HEART DISEASE
In infants CHD may cause HF through three general mechanisms: critical left-sided obstruction, valvular regurgitation, and left-to-right shunts, any of which may coexist in an individual patient. Critical left-sided obstructive lesions require patency of the ductus ateriosus postnatally to maintain adequate cardiac output, as in hypoplastic left heart syndrome (HLHS) or critical coarctation of the aorta. Neonates with critical left heart obstruction may be relatively asymptomatic and have an unremarkable physical examination in the first few days after birth. Should the underlying disease go unrecognized, closure of the ductus arteriosus will lead to tachypnea, poor feeding, irritability, and ultimately signs of shock in a presentation that may mimic sepsis. Initiation of prostaglandin infusion will often restore ductal patency and may be lifesaving. Routine newborn screening with pulse oximetry prior to hospital discharge, if properly performed, will readily identify infants with critical left heart obstruction because obligatory right to left shunting at the ductus arteriosus results in differential cyanosis (normal oxygen saturations in the right upper extremity and depressed oxygen saturations in either lower extremity).2 It should be noted that critical right heart obstructive lesions (i.e., pulmonary atresia) generally present with profound cyanosis not with HF.
Valvular regurgitation may result in HF by limiting forward cardiac output and causing pulmonary congestion, as in the example of congenital mitral insufficiency. Chronic severe valvular regurgitation leads to ventricular dysfunction once the ventricle’s ability to compensate for reduced forward output by increasing stroke volume is compromised. Severe tricuspid regurgitation in the setting of Ebstein’s abnormality of the tricuspid valve can also cause HF symptoms in the neonate, which primarily manifests as venous congestion and in severe cases, low cardiac output due to the inability of the right ventricle to propel sufficient blood through the pulmonary vascular bed. CHD associated with defects that lead to excessive left-to-right shunting may also lead to symptoms that mimic CHF. Examples of such conditions include large ventricular septal defect, complete common atrioventricular canal defect, and large patent ductus arteriosus. The use of the term “heart failure” in this scenario is actually somewhat of a misnomer, since there is usually no associated ventricular dysfunction or limitation to cardiac output. Rather, the symptoms of excessive left-to-right shunting mimic a HF syndrome by causing tachypnea, diaphoresis, and failure to thrive because pulmonary blood flow is far in excess of systemic blood flow, which directly results in pulmonary congestion.
A rare but important congenital heart defect that may manifest as HF with a dilated cardiomyopathy in infants is anomalous origin of the left coronary from the pulmonary artery (ALCAPA).3 In the early neonatal period when pulmonary arterial pressures are high, coronary perfusion pressure is normal and the myocardium is not adversely affected by perfusion with poorly saturated blood. However, as pulmonary vascular resistance and pulmonary artery pressure decline over the first few weeks of life, coronary perfusion falls and flow in the left coronary artery can even reverse. Gradual ischemia develops in the myocardium perfused by the anomalous left coronary artery ensues, leading to ventricular dilation, mitral regurgitation, and eventually symptomatic ventricular dysfunction. Recognition of this defect as the underlying cause of cardiomyopathy is of paramount importance because the cardiomyopathy and resulting HF syndrome may be largely reversed by prompt surgical revascularization of the ischemic myocardium.
In older children, HF related to CHD is most often related to sequelae of prior surgical or transcatheter interventions for CHD, since it is rare in developed countries for HF related to “unoperated” CHD to present later in childhood. An example is systolic ventricular dysfunction in patients with a systemic right ventricle, as seen in HLHS following the Norwood procedure, or in transposition of the great arteries following an atrial switch (Senning or Mustard) procedure. In such patients the “new” systemic right ventricle, which is better suited morphologically for pumping into a low-pressure vascular bed, may exhibit progressive systolic ventricular dysfunction with chronic exposure to higher (systemic) afterload. Other commonly encountered examples include right ventricular dysfunction in patients who have had a transannular patch repair of tetralogy of Fallot (in which a ventriculotomy is a necessary part of the procedure), as well as ischemic left ventricular dysfunction late following the arterial switch operation for transposition of the great arteries (in which necessary mobilization of the coronary arteries may lead to coronary narrowing or distortion). Because the symptoms of HF related to such interventions may not manifest until late adolescence or early adulthood, referral of patients who have had previous interventions for CHD to adult cardiology providers experienced in CHD is essential.
CARDIOMYOPATHIES
Cardiomyopathy, or disease intrinsic to the cardiac muscle, may be divided into five categories based on pathophysiology and shared clinical characteristics: dilated cardiomyopathy, hypertrophic cardiomyopathy, restrictive cardiomyopathy, left ventricular noncompaction cardiomyopathy, and arrhythmogenic right ventricular dysplasia/cardiomyopathy. (The latter is exceedingly rare in children and will not be discussed further.)
Dilated cardiomyopathy is the most common cardiomyopathy in children, with an annual incidence of approximately 0.6 cases per 100,000 children per year.4 It is typically idiopathic in etiology, although it may be follow an inflammatory process such as myocarditis, and has a familial component in 30%-40% of cases.5 Patients may present with indolent symptoms such as fatigue, failure to thrive, or tachypnea, but arrhythmias and sudden death are important presenting signs in a minority. Of interest, gastrointestinal symptoms are frequent in patients presenting with a new diagnosis of cardiomyopathy; a high index of suspicion is required when evaluating patients with chronic abdominal complaints that have no readily identifiable cause.6 In boys, dilated cardiomyopathy is a nearly universal finding in the Becker and Duchenne muscular dystrophies, with cardiomyopathy typically becoming clinically apparent in the early teenage years and being the proximate cause of death in most patients in the early 20s. Neonates and infants, on account of their inability to relate symptoms, are particularly susceptible to dilated cardiomyopathy from prolonged, unrecognized tachyarrhythmias (tachycardia-induced cardiomyopathy).
Hypertrophic cardiomyopathy rarely causes symptoms of HF in children, but it is the most common cause of sudden death in adolescents and young adults so it is important to exclude this diagnosis in patients at risk for the disease.7 The means and extent to which athletes should undergo screening for hypertrophic cardiomyopathy prior to participating in competitive sports remains controversial, although the American College of Cardiology and American Heart Association recently released guidelines for clinicians.8 Like dilated cardiomyopathy, hypertrophic cardiomyopathy also has a strong familial component, and first degree relatives of patients with either entity should undergo cardiac evaluation including an EKG and echocardiogram. Hypertrophic cardiomyopathy is also encountered in infants with diabetic mothers as well as those with certain genetic syndromes, most notably Noonan syndrome.
Restrictive cardiomyopathy and left ventricular noncompaction cardiomyopathy are occasionally encountered in children. In restrictive cardiomyopathy, systolic ventricular function is preserved, the ventricle is not dilated, and the primary pathologic mechanism is impaired diastolic filling. There is a high incidence of pulmonary hypertension and sudden death, with few options for medical management, so transplantation is considered when the diagnosis is made.9
Left ventricular noncompaction cardiomyopathy can be characterized as an arrest of embryologic development of the left ventricular myocardium, in which the normally smooth surface of the endocardium is replaced by a densely trabeculated endocardial surface. Patients frequently have an undulating clinical course, with thromboembolic events and arrhythmias as notable features unique to the diagnosis.
Myocarditis, defined as lymphocytic infiltration of the myocardium with an associated inflammatory response, is an important cause of HF in children. It may be idiopathic, but several infectious agents (primarily viral) are commonly encountered in serum or in myocardial biopsies, including parvovirus, coxsackievirus, adenovirus, and human herpesvirus-6. Clinically, myocarditis may be differentiated from dilated cardiomyopathy by the acute onset of symptoms, a recent history of a viral upper respiratory tract infection, elevation of serum inflammatory markers, and the presence of pericardial effusion on echocardiography. Nonetheless, there is often a broad overlap, and it is not always easy to distinguish between the two.
ACQUIRED HEART DISEASE
Rheumatic heart disease (RHD) is the most common cause of acquired heart disease in children and young adults in developing countries. In the primary phase of illness, RHD manifests as a valvulitis, with the mitral and/or aortic valves affected most commonly. In the acute phase of the disease valvular regurgitation may lead to HF; left untreated, chronic valvular regurgitation and/or stenosis may develop. With prompt recognition and treatment of uncomplicated S. pyogenes infection, acute and chronic RHD is rare in the US, but outbreaks do continue to occur in sporadic clusters in specific populations.
Kawasaki disease (KD) is the most common cause of acquired heart disease in developed countries, including the U.S. Its hallmark is the development of coronary artery ectasia and/or aneurysms in approximately 25% of patients following the acute phase of illness; this risk is reduced to <5% with the administration of immune globulin.10 However, KD can directly cause carditis during the acute phase of illness, which may result in symptoms of acute HF. In those with coronary artery aneurysms, the risk of coronary thrombosis and/or dissection is significant, and these may lead to acute and chronic HF.
Children treated for various malignancies with anthracycline chemotherapy (doxorubicin, daunorubicin) have a lifelong risk of developing cardiotoxicity, which most commonly manifests as dilated cardiomyopathy.11 The overall risk is 10%; factors that increase the risk include younger age at time of chemotherapy, higher cumulative dose, and concomitant receipt of radiation to the chest. Since the risk is lifelong, routine echocardiographic followup for survivors of childhood cancer should continue well into adulthood.
EVALUATION OF CHILDREN WITH SUSPECTED HEART FAILURE
Although physical examination findings are rarely specific, close attention will often provide insights into the presence and cause of HF. Heart rate is a critically important measure of cardiovascular health, and patients with new-onset dilated cardiomyopathy or myocarditis may present with “inappropriate” sinus tachycardia. Blood pressure is usually normal in patients with HF; hypotension is a late finding in decompensated HF and usually portends impending cardiovascular collapse. Other important aspects of the physical examination of the child with HF include respiratory pattern and effort; presence of a gallop on auscultation; adequacy of peripheral perfusion as judged by skin temperature, capillary refill and amplitude of the peripheral pulses; and intravascular fluid status as indicated by hepatomegaly, venous distension, and peripheral edema. The clinical assessment of patients with HF can help place them into categories according to their state of hemodynamic compensation, pulmonary congestion, and peripheral perfusion, all of which can help direct initial management (Table 2).
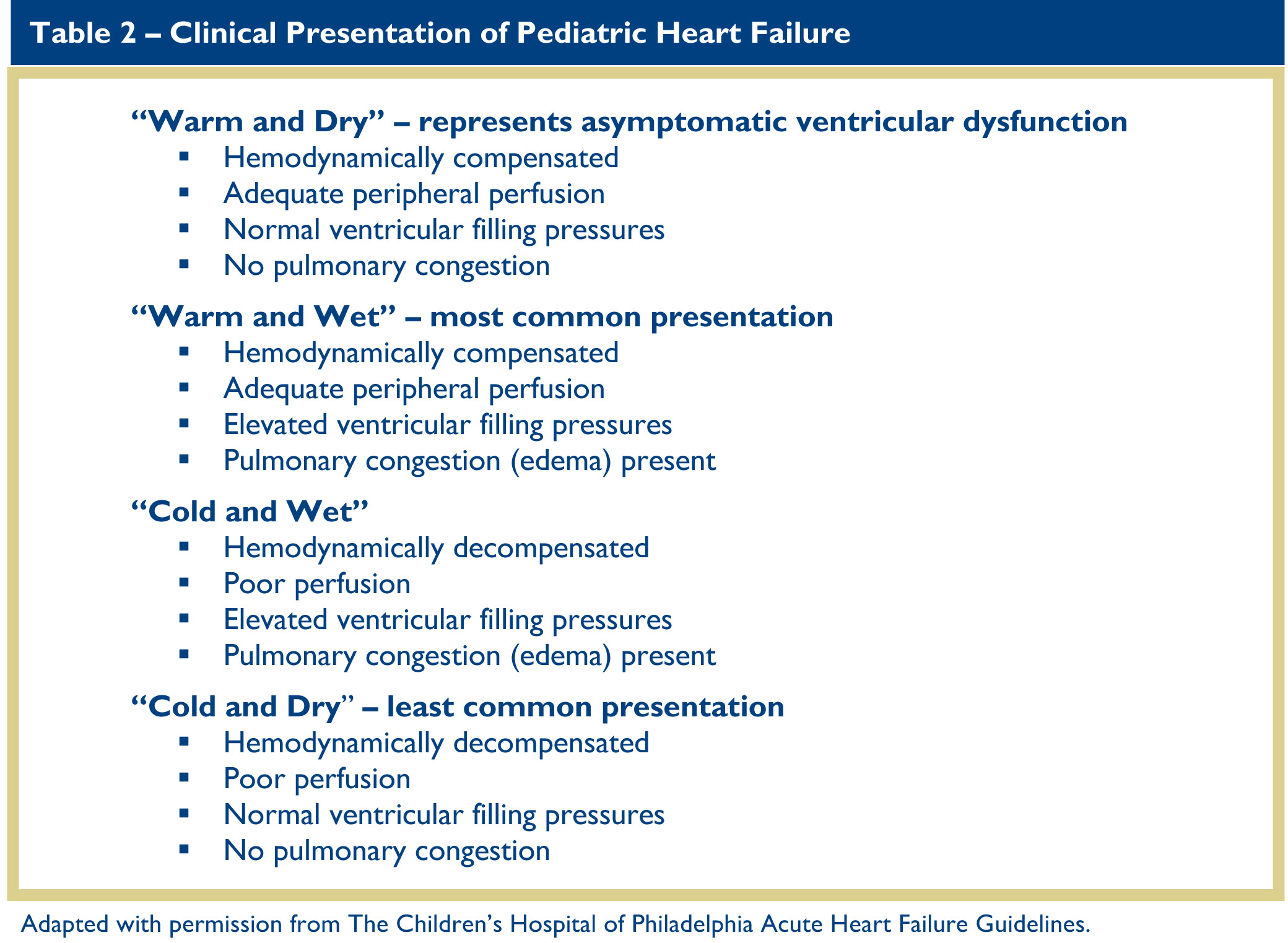
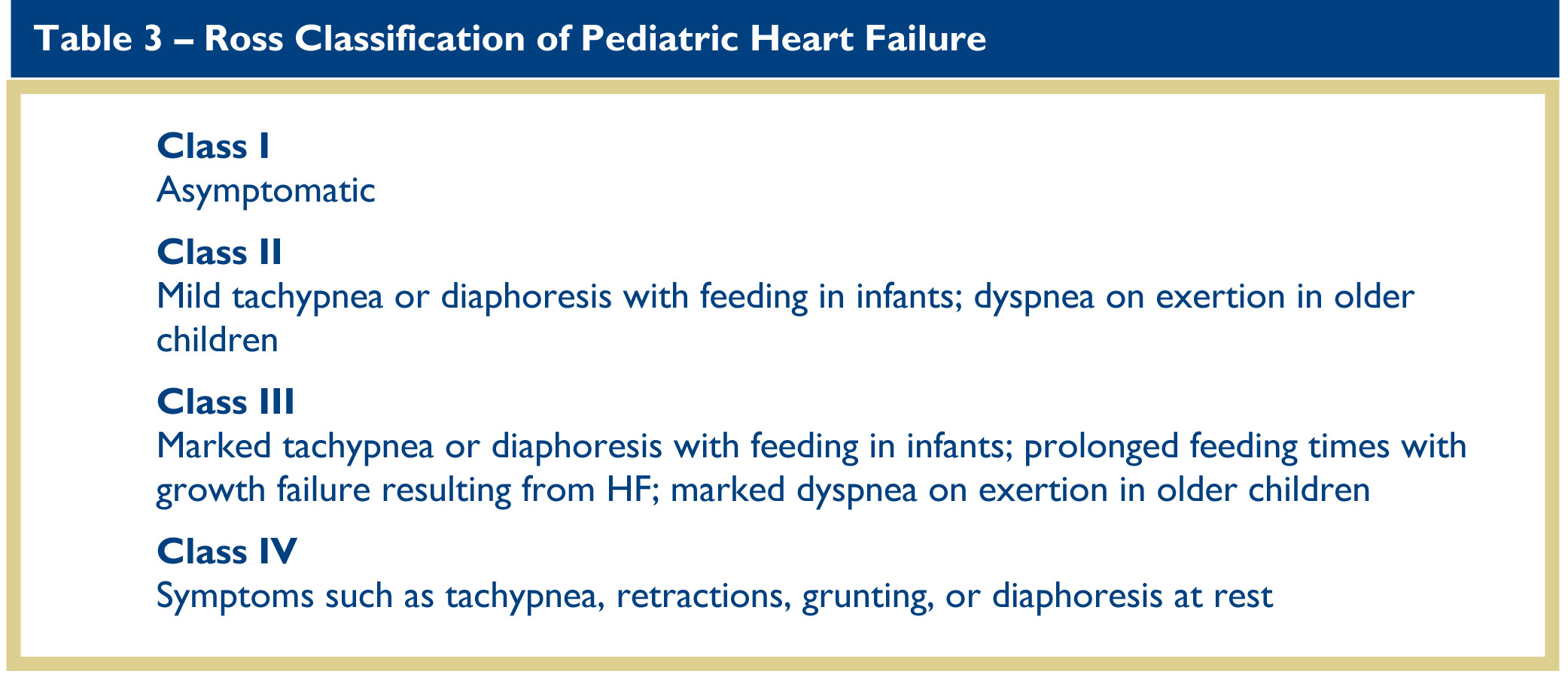
Assessment of the history obviously must be tailored to the age and developmental status of the patient. In infants, assessment of HF focuses on feeding habits. In children, particularly if they are too young to relate “classic” symptoms of HF such as chest pain, palpitations, or dyspnea, assessment of their level of activity is more useful, especially their willingness to participate in activities they habitually enjoyed. Similar to the well-known New York Heart Association clinical severity scale in adults with HF, the Ross classification of pediatric HF symptoms defines severity with respect to normal, age-appropriate, childhood activities (Table 3).12
In addition to the chest radiograph and electrocardiogram, which are essential in the evaluation of any child with suspected HF, noninvasive cardiac imaging plays a key role in the diagnosis of suspected HF. Complete 2-dimensional echocardiography with Doppler is usually sufficient for the diagnosis of most cardiomyopathies, except for arrhythmogenic right ventricular dysplasia/cardiomyopathy. In addition, echocardiography can readily identify and semi-quantitatively assess valvular regurgitation and shunts, and can provide functional information such as ejection fraction, ventricular dimensions, and estimates of right ventricular systolic pressure, but it has a limited role in characterizing myocardial tissue and may not provide sufficient visualization of the requisite cardiac structures in older and/or obese patients.
Cardiac magnetic resonance imaging is playing an increasingly important role in addressing the limitations of echocardiography , but it requires sedation in most children under 12 years. Invasive hemodynamic assessment (cardiac catheterization) is limited to cases in which the diagnosis is uncertain or in which quantitative measurement of hemodynamic parameters such as cardiac output and pulmonary capillary wedge pressure are necessary, particularly if a patient is being considered for heart transplantation.
The laboratory evaluation of a child with suspected HF serves three purposes: it can confirm the diagnosis, provide information about the severity of illness, and exclude other confounding diagnoses. At a minimum, children with suspected HF should have measurement of serum electrolytes and hepatic function, a complete blood count with differential, and thyroid hormone assessment. Frequent monitoring of electrolytes and renal function is necessary in children receiving diuretics and ACE inhibitors. A comprehensive metabolic evaluation and consultation with an expert in metabolism is mandatory in infants with HF, since many inborn errors of metabolism are associated with cardiomyopathy (e.g. Pompe disease).
Biomarkers also play an important role in the assessment of HF in children. B-natriuretic peptide (BNP), released by ventricular myocardium in response to myofibril stretch, is a reasonably sensitive and specific adjunct to the diagnosis of HF in children and adults.13-15 In particular, an elevated BNP can help distinguish HF from respiratory disease. Serial measurement of BNP in patients with known HF is also useful for assessing exacerbations of HF, monitoring response to therapy, and evaluating prognosis. Care must be taken when comparing BNP values measured at different hospitals or institutions, as different normative values exist specific to the individual proprietary assay used. In addition, many centers follow the N-type-proBNP, a precursor to BNP, but the two measurements are not directly comparable. Serum troponin is also an important biomarker in children. Elevated levels are observed in cardiac ischemia and in inflammatory processes such as myocarditis, and can help distinguish such diagnoses from primary cardiomyopathies.
TREATMENT OF HEART FAILURE
The clinical severity of HF in children ranges from asymptomatic states manifested only by ventricular dysfunction on echocardiogram to sudden cardiac death, so the intensity of therapy is generally tailored to the patient’s symptoms and functional state, though with several exceptions. In general, management strategies for pediatric HF reflect extrapolation of experience and results from adult data, as the number of high quality studies examining the effect of HF therapies specifically in children is quite limited.16 Much of this lack of knowledge
derives from the rarity and heterogeneity of HF in children, but other factors involve reluctance to involve children in research as well as the limited return on investments in expensive randomized trials that may only benefit a very small segment of the population.
Children with HF symptoms secondary to CHD require surgical and/or transcatheter correction of the defect, or palliation when an anatomic and physiologic correction is not feasible. While it is common to stabilize children with ductal-dependent left heart disease for some period of time after diagnosis, there is little role for prolonged medical management if there are no contraindications to surgery. Similarly, infants with a HF “syndrome” in the setting of large left-to-right shunts may be medically managed with diuretics and nasogastric feedings to relieve symptoms and optimize nutrition. However, the widespread availability of neonatal cardiac surgery and data demonstrating excellent outcomes for infants as low as 2 kg argue against prolonged periods of medical management.
For adults with symptomatic ventricular dysfunction, the mainstay of outpatient oral medical therapy involves the combination of a beta-blocker, an ACE inhibitor (or angiotensin receptor blocker), and an aldosterone antagonist. For symptomatic relief, digoxin and diuretics are frequently utilized, but neither of these medications has been shown to offer a survival benefit. In children with symptomatic systolic ventricular dysfunction the combination of beta-blocker, ACE inhibitor, and aldosterone antagonist is also frequently employed, even though the only randomized, controlled trial of a HF medication (carvedilol) in children failed to demonstrate any benefit relative to placebo.17 For children with refractory HF symptoms despite optimal outpatient management, hospitalization is necessary and frequently involves the use of inotropic medications to treat hypotension and/or refractory symptoms.
HEART TRANSPLANTATION
The definitive therapy for refractory HF in adults and children is heart transplantation. The decision to commit a child to heart transplantation is complex; the uncertainties about optimal timing are compounded by the expectation that a heart transplant during childhood will not serve a child’s expected lifespan. Common indications for heart transplantation involve refractory HF symptoms, growth failure in the setting of HF, CHD without options for surgical repair or palliation, and the diagnosis of restrictive cardiomyopathy. The current outcomes of heart transplantation in children are generally favorable, and the expected half-life (the time at which 50% of patients remain alive with the original graft) in the current era is approaching 20 years.18 Moreover, children (and their families) who have undergone heart transplantation report excellent quality of life measures and are generally indistinguishable from their peers with respect to activities of daily living.
MECHANICAL ASSIST DEVICES
The management of HF in adults has been revolutionized by the widespread adoption of durable ventricular assist devices (VADs) for clinical stabilization and rehabilitation prior to transplantation or for “destination therapy.” A prior limitation to the use of VADs in children was lack of devices that are small enough, particularly for infants and younger children. However, after several years of experience in Europe, the FDA in 2011 approved the first pediatric-specific VAD: the Berlin Heart EXCOR VAD.19 This pulsatile device is available in several different sizes approximating normal pediatric stroke volumes, which can permit the use of long-term support in children as small as 3 kg. Newer continuous-flow devices such as the HeartWare VAD are being used as bridge-to-transplant with increasing frequency in children as small as 0.7 m2 body surface area, including at CHOP. The concept of destination therapy in pediatrics has not been widely adopted, but has been explored in patients with Duchenne muscular dystrophy.
PROGNOSIS
The prognosis for HF in children is variable and highly independent of the etiology. For relatively common causes such as myocarditis and dilated cardiomyopathy, a surprisingly high incidence of spontaneous normalization of heart function has been observed in several large studies.17,20 In the randomized trial of carvedilol, for example, spontaneous improvement was noted in nearly 60% of the placebo group. Hypertrophic cardiomyopathy generally has a favorable prognosis during childhood, with a low incidence of sudden death or deterioration in ventricular function.21 As a rule, children with cardiomyopathy are not restricted from activities, apart from competitive sports, and are encouraged to participate in activities up to their individual ability and endurance.
CONCLUSION
Relative to the adult population, the incidence of HF in children is rare. The etiologies of HF in children overlap those seen in adults, although CHD is an important and distinct contributor to etiology. The treatment of HF in children mirrors strategies employed in the adult population, and it is anticipated that ongoing advances in adult HF will translate into improved outcomes for children. Several professional societies have published recently updated guidelines for the management of HF in children, which can aid the clinician interested in learning more about the most up-to-date therapies and outcomes for this patient population.22,23
REFERENCES
1. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013;128:e240-327.
2. Kemper AR, Mahle WT, Martin GR, Cooley WC, Kumar P, Morrow WR, et al. Strategies for implementing screening for critical congenital heart disease. Pediatrics 2011;132:e185-e192.
3. Wesselhoeft H, Fawcett JS, Johnson AL. Anomalous origin of the left coronary artery from the pulmonary trunk. Its clinical spectrum, pathology, and pathophysiology, based on a review of 140 cases with seven further cases. Circulation 1968;38:403-25.
4. Towbin JA, Lowe AM, Colan SD, Sleeper LA, Orav EJ, Clunie S, et al. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA 2006;296:1867-76.
5. Hershberger RE, Siegfried JD. Update 2011: clinical and genetic issues in familial dilated cardiomyopathy. J Am Coll Cardiol 2011;57:1641-9.
6. Macicek SM, Macias CG, Jefferies JL, Kim JJ, Price JF. Acute heart failure syndromes in the pediatric emergency department. Pediatrics 2009;124:e898-904.
7. Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011;124:e783-831.
8. MS. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 2: preparticipation screening for cardiovascular disease in competitive athletes: a scientific statement from the American Heart Association and American College of Cardiology. J Am Coll Cardiol 2015;66:2356-61.
9. Webber SA, Lipshultz SE, Sleeper LA, Lu M, Wilkinson JD, Addonizio LJ, et al. Outcomes of restrictive cardiomyopathy in childhood and the influence of phenotype: a report from the Pediatric Cardiomyopathy Registry. Circulation 2012;126:1237-44.
10. Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation 2004;110:2747-71.
11. Lipshultz SE, Adams MJ, Colan SD, Constine LS, Herman EH, Hsu DT, et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association. Circulation 2013;128:1927-95.
12. Ross RD. The Ross classification for heart failure in children after 25 years: a review and an age-stratified revision. Pediatr Cardiol. 2012;33:1295-1300.
13. Auerbach SR, Richmond ME, Lamour JM, Blume ED, Addonizio LJ, Shaddy RE, et al. BNP levels predict outcome in pediatric heart failure patients: post hoc analysis of the Pediatric Carvedilol Trial. Circ Heart Fail 2010;3:606-11.
14. Maher KO, Reed H, Cuadrado A, Simsic J, Mahle WT, Deguzman M, et al. B-type natriuretic peptide in the emergency diagnosis of critical heart disease in children. Pediatrics 2008;121:e1484-8.
15. Palazzuoli A, Gallotta M, Quatrini I, Nuti R. Natriuretic peptides (BNP and NT-proBNP): measurement and relevance in heart failure. Vasc Health Risk Manag 2010;6:411-8.
16. Rossano JW, Shaddy RE. Update on pharmacological heart failure therapies in children: do adult medications work in children and if not, why not? Circulation 2014;129:607-12.
17. Shaddy RE, Boucek MM, Hsu DT, Boucek RJ, Canter CE, Mahony L, et al. Carvedilol for children and adolescents with heart failure: a randomized controlled trial. JAMA 2007;298:1171-9.
18. Dipchand AI, Edwards LB, Kucheryavaya AY, Benden C, Dobbels F, Levvey BJ, et al. The registry of the international society for heart and lung transplantation: seventeenth official pediatric heart transplantation report-2014; focus theme: retransplantation. J Heart Lung Transplant 2014 ;33:985-95.
19. Fraser CD,Jr, Jaquiss RD, Rosenthal DN, Humpl T, Canter CE, Blackstone EH, et al. Prospective trial of a pediatric ventricular assist device. N Engl J Med 2012;367:532-41.
20. Alexander PM, Daubeney PE, Nugent AW, Lee KJ, Turner C, Colan SD, et al. Long-term outcomes of dilated cardiomyopathy diagnosed during childhood: results from a national population-based study of
childhood cardiomyopathy. Circulation 2013;128:2039-46.
21. Maron BJ, Rowin EJ, Casey SA, Lesser JR, Garberich RF, McGriff DM, et al. Hypertrophic cardiomyopathy in children, adolescents, and young adults associated with low cardiovascular mortality with contemporary management strategies. Circulation 2016;133:62-73.
22. McKelvie RS, Moe GW, Ezekowitz JA, Heckman GA, Costigan J, Ducharme A, et al. The 2012 Canadian Cardiovascular Society heart failure management guidelines update: focus on acute and chronic heart failure. Can J Cardiol 2013;29:168-81.
23. Kirk R, Dipchand AI, Rosenthal DN, Addonizio L, Burch M, Chrisant M, et al. The International Society of Heart and Lung Transplantation guidelines for the management of pediatric heart failure: executive summary. J Heart Lung Transplant 2014;33:888-909.