 Click to Print Adobe PDF
Click to Print Adobe PDF
Winter 2015 - Vol. 10, No. 4
|
Transcatheter Aortic Valve Replacement:
Review and LGH Experience
James E. Harvey, M.D., M.Sc.
Medical Director, Structural Heart Intervention
Sherri S. Delgado, M.S.N., C.R.N.P., C.C.R.N.
LGH Structural Heart Coordinator
|
  |
BACKGROUND
Ten percent to twenty percent of all cardiac operations in the United States are done for valvular heart disease, and aortic valve replacement comprises two-thirds of valve operations. Degenerative aortic stenosis (AS) is the most common form of valvular heart disease in developed countries, and in the U.S., the majority of aortic operations are for AS.1
The prevalence of AS increases significantly with age. Moderate to severe aortic stenosis is found in only 0.02% of people aged 18 to 44 years, but it increases to 2.8% in people aged 75 years or older.2 Patients with AS typically have a long latent phase during which they are asymptomatic,3 but once symptoms develop they have a poor prognosis; with medical therapy alone survival rates are as low as 50% at 2 years and 15% at 5 years.4
Traditionally, patients with severe, symptomatic AS are treated with surgical aortic valve replacement (SAVR), which entails sternotomy, cardiopulmonary bypass, excision of the entire diseased valve, and suture of a mechanical or bioprosthetic valve to the aortic annulus. But though SAVR significantly decreases mortality in patients with symptomatic aortic stenosis and it significantly improves their quality of life,5 many patients with AS are unfortunately deemed too sick to undergo SAVR and are not offered surgery.6 Patients considered too high a risk to undergo SAVR have a much worse prognosis, and less than one third survive beyond 2 years.7
THE EMERGENCE OF TAVR
Transcatheter aortic valve replacement (TAVR) has emerged as an alternative to surgery for patients with severe, symptomatic aortic stenosis. The first transcatheter aortic valve implantation was performed by Dr. Alain Cribier in April 2002.8 Since then, advances in equipment and technique have led to an exponential increase in the worldwide utilization of TAVR, and by 2013 over 80,000 procedures had been performed.9
In TAVR, a bioprosthetic valve is mounted on a catheter, advanced to the aortic annulus, then expanded and deployed inside the native diseased valve [Figures 1 and 2].
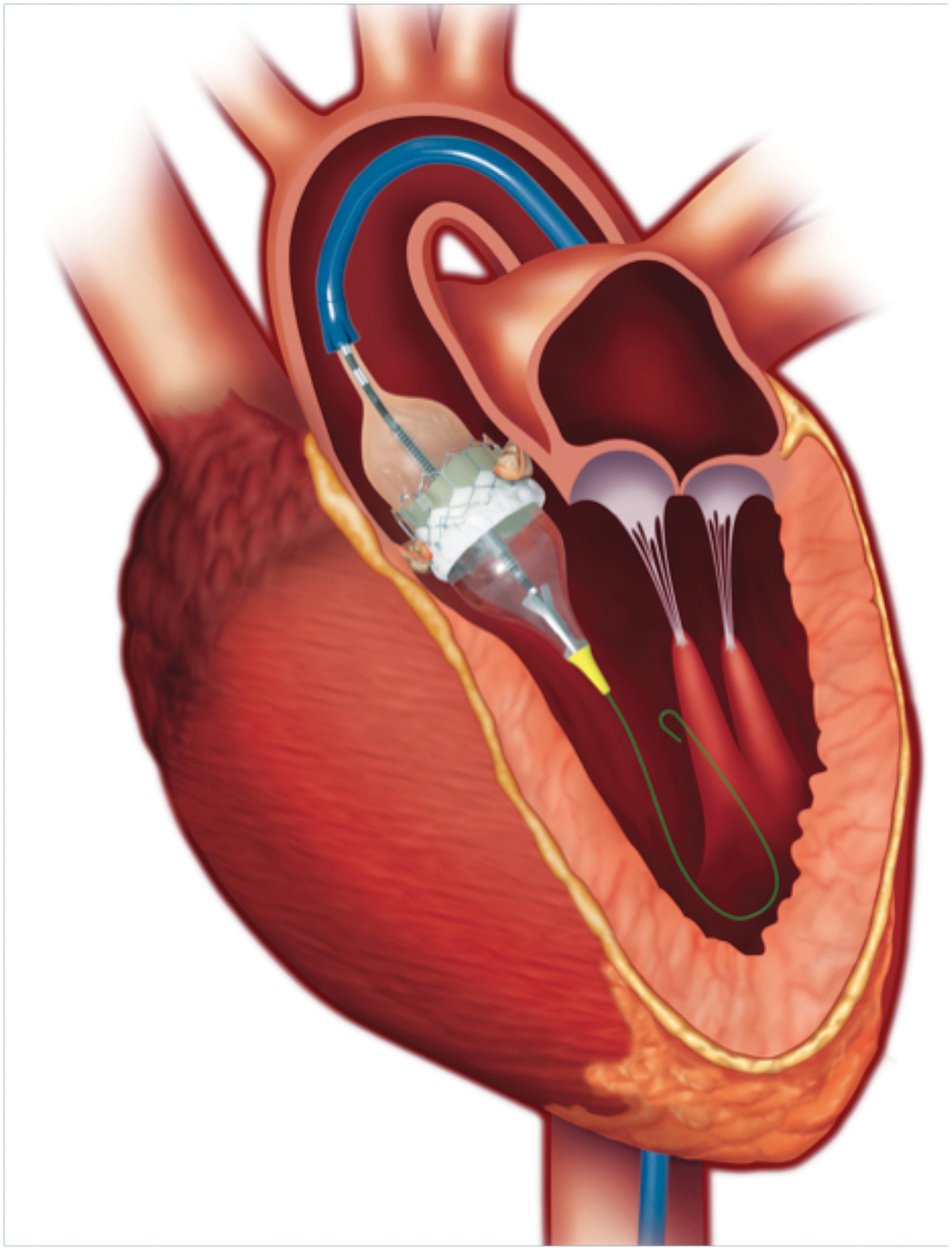
Fig. 1. An Edwards Sapien 3 balloon expandable valve being deployed in the aortic annulus in a patient with severe aortic stenosis. The catheter has been advanced from the common femoral artery access site. (Edwards LifeSciences Inc.)
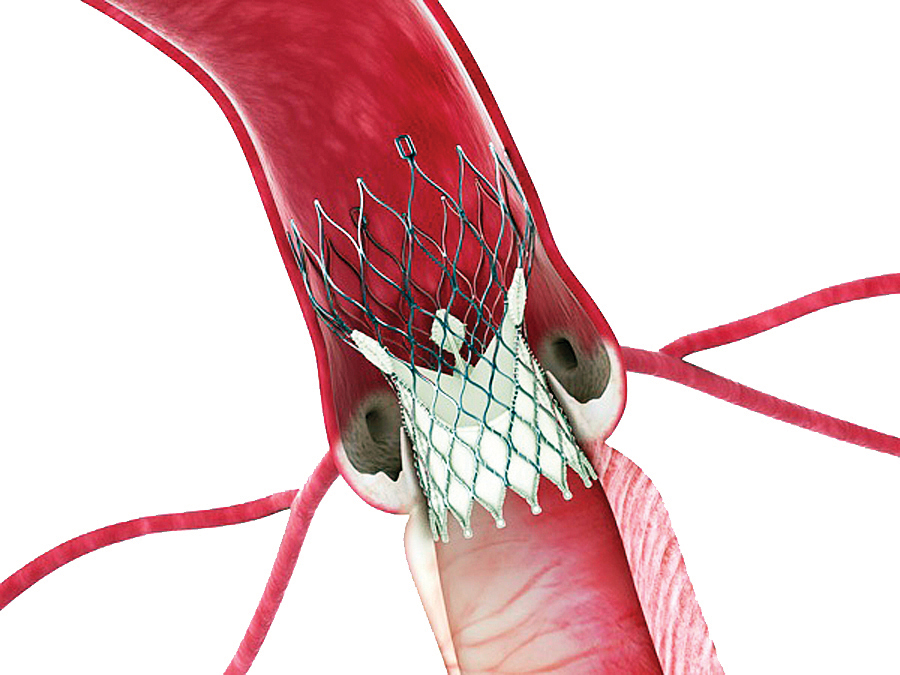
Fig. 2. A Medtronic CoreValve self-expanding valve deployed in the aortic annulus in a patient with severe aortic stenosis. (Medtronic Inc.)
Current transcatheter valves consist of a tissue valve made from bovine or porcine pericardium attached to a cylindrical metal cage. In most patients, arterial access is obtained at the common femoral artery and the valve is delivered to the aortic annulus through the iliac artery and aorta. However, in patients with inadequate peripheral arteries, alternative routes of delivery include the subclavian artery, the ascending aorta via a small upper sternotomy, or directly through the left ventricular apex via a small lateral thoracotomy.
There are two types of transcatheter valves approved for commercial use in the United States: Balloon Expandable Valves (BEV) and Self Expanding Valves (SEV). The SAPIEN valve, developed by Edwards Lifesciences (Irvine, CA), is a BEV and was the first valve commercially available in the U.S. in November 2011. In the Placement of Aortic Transcatheter Valves (PARTNER) trial, TAVR with the SAPIEN valve resulted in a 25% absolute reduction in mortality at 2 years when compared to medical therapy alone (43.3% for TAVR vs. 68.0% for medical therapy, p < 0.01).7 When compared to SAVR, TAVR resulted in similar 2-year mortality rates in patients determined to be at high surgical risk (33.9% two year mortality for TAVR vs. 35.0% for SAVR, p = 0.78).10
In January 2014, the self-expanding Medtronic CoreValve was approved for TAVR in the United States. The CoreValve US Pivotal Trial demonstrated its efficacy and safety, with a 15.9% absolute reduction in 1 year mortality in patients deemed at extreme risk for SAVR, compared with medical therapy alone (8.4% for TAVR vs. 24.3% for medical therapy, p < 0.01).11 In addition, in patients deemed merely high risk for surgery, TAVR with the CoreValve resulted in a 4.9% absolute reduction in 1 year mortality compared with SAVR (14.2% for TAVR vs. 19.1% for SAVR, p = 0.04 for superiority).12 TAVR with both the Sapien and CoreValve resulted in decreased hospital length of stay and improved quality of life at 30 days when compared to SAVR.
EARLY CHALLENGES AND RECENT ADVANCES
The early success of TAVR was not without challenges. The first TAVR delivery platforms utilized catheters with an inner diameter of 22 to 24 French (7.3 – 8 mm), which resulted in an initial 30-day rate of peripheral vascular complication of 16.8%.13 In addition, for patients deemed to have prohibitive surgical risk the 30-day rate of stroke was 5.0% with TAVR vs. 1.1% with only medical therapy. The rate of moderate or greater paravalvular aortic insufficiency at 1 year with TAVR was 4.3-10.5% and the 30-day rate of significant conduction abnormality requiring pacemaker implantation ranged from 3.4% with BEV to 21.6% with SEV.11,13
Fortunately, advances in technology and procedural technique led to significant improvements in clinical outcomes and reductions in complication rates. The original Edwards Sapien valve was modified, and the Sapien XT allowed a smaller delivery catheter and larger valve sizes.14 It also proved to be an effective therapy for treatment of failed surgical bioprostheses.15 Recently, the S3 transcatheter valve was released, which can be delivered through a 14 to 16 Fr arterial sheath and accommodates an aortic annulus size from 18 to 28 mm. This development allows a much higher percentage of patients to undergo TAVR via femoral artery access. The S3 has an external cuff that has greatly minimized paravalvular leak (PVL), such that only 2.7% of moderate PVL and no severe PVL are reported [Figure 3].

Fig. 3. The S3 transcatheter valve. The external cuff around the inferior aspect of the valve minimizes paravalvular leak. (Edwards LifeSciences Inc.)
In addition, the rates of disabling stroke and all-cause mortality at 30 days are 0.9% and 2.2%, respectively.16
The self-expanding CoreValve has also been modified, giving rise to the Evolut R transcatheter valve [Figure 4].
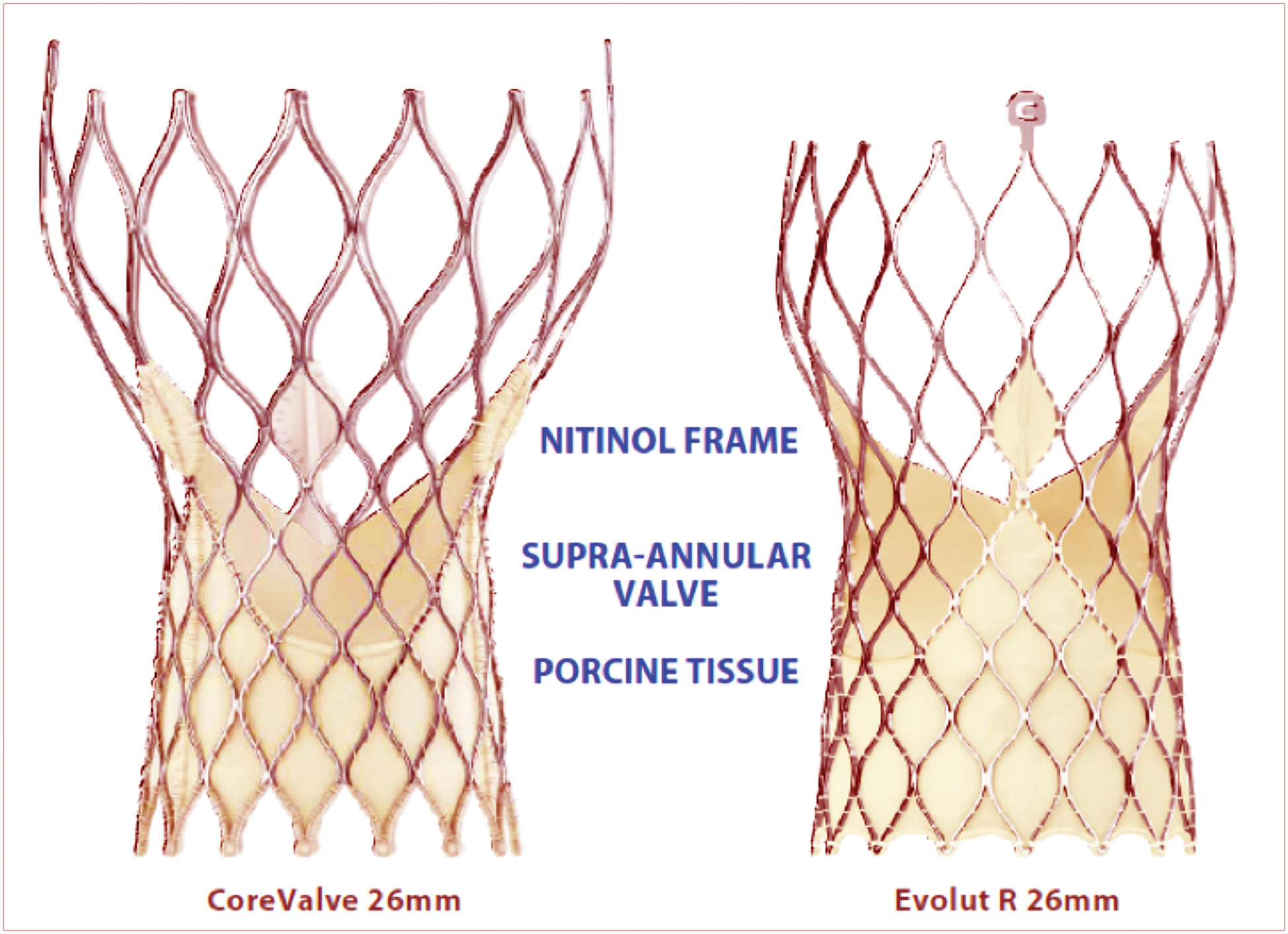
Fig. 4. A 26 mm CoreValve and a 26 mm Evolut R. Both valves consist of a tissue valve made of porcine pericardium sewn to a nitinol frame. (Medtronic Inc.)
This valve also demonstrates a low rate of PVR at 30 days: 3.4% moderate and 0% severe. More importantly, the valve is completely repositionable and demonstrates a 0% rate of stroke or death at 30 days.17 The rate of permanent pacemaker implantation with this device has improved, but is still 11.7% at 30 days.
THE NEED FOR FURTHER DEVELOPMENT
Despite the recent advances in TAVR, there are several areas in need of improvement. According to international registries of TAVR, major vascular complications still occur in 2% to 13% of cases18 and permanent pacemaker implantation is performed after 5% to 50%.19,20 Although there has been a significant reduction in the occurrence of stroke and PVL, continued improvement is needed in these areas as well. Several new devices are in various stages of development and may potentially address some of these issues. The Portico transcatheter valve (St. Jude Medical Inc., St. Paul, Minnesota) and the Lotus transcatheter valve (Boston Scientific Inc., Marlborough, MA) have both received CE approval in Europe and are undergoing evaluation in the U.S. [Figure 5].
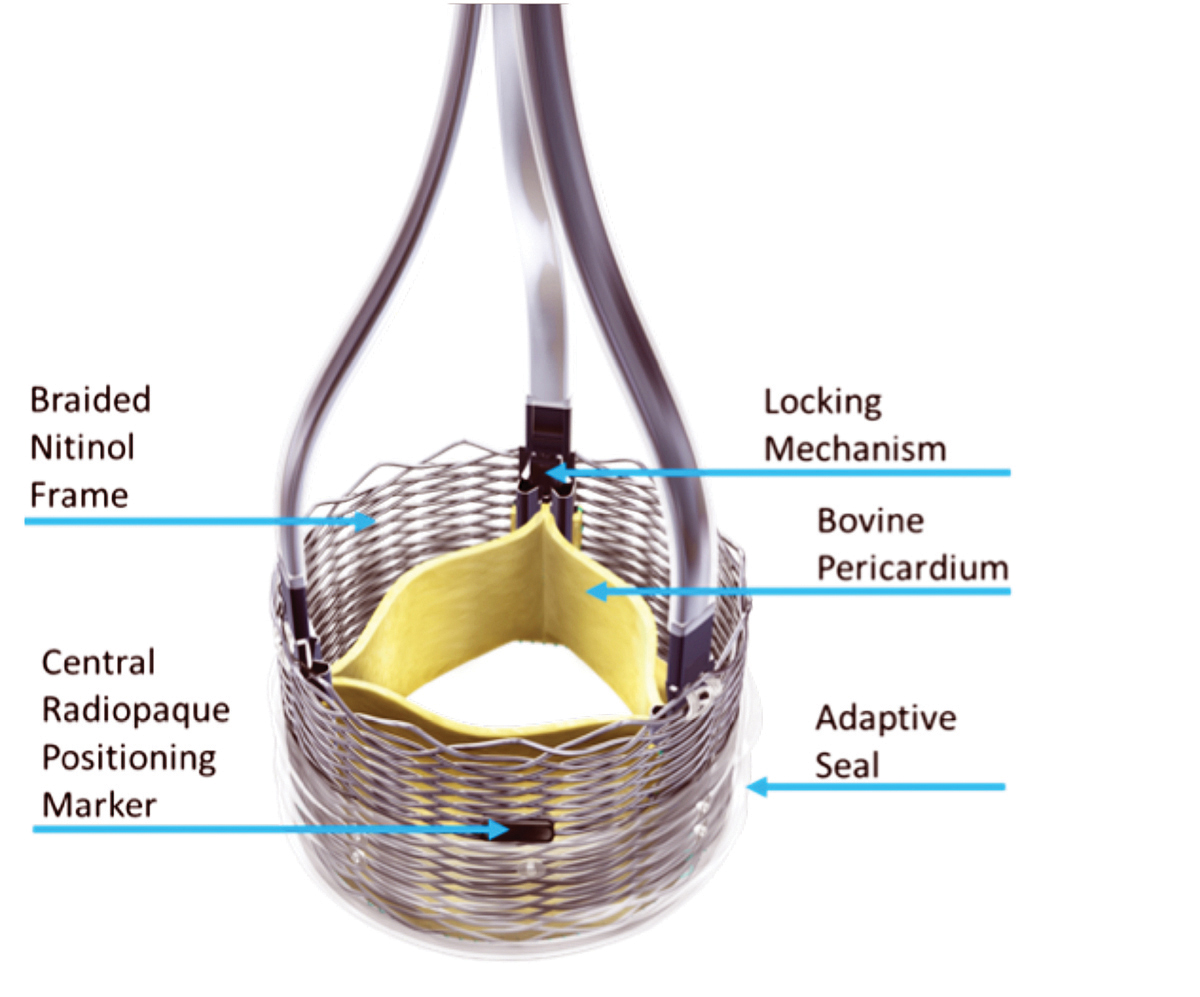
Fig. 5. The Lotus transcatheter valve consists of a tissue valve made from bovine pericardium attached to a braided nitinol frame. It can be retrieved and repositioned after complete deployment and functionality. This allows for a complete assessment prior to deciding to permanently release the valve. (Boston Scientific Inc., Marlborough, MA)
Additionally, there are ongoing studies evaluating the safety and efficacy of TAVR in unstudied patient populations including those with moderate surgical risk. The results of these studies could broaden the indications for TAVR.
THE LGH EXPERIENCE
LGH performed its first TAVR in August 2012. Since that time, the program has grown considerably and the hospital will have performed more than 120 TAVRs at the time of this publication [Figure 6].
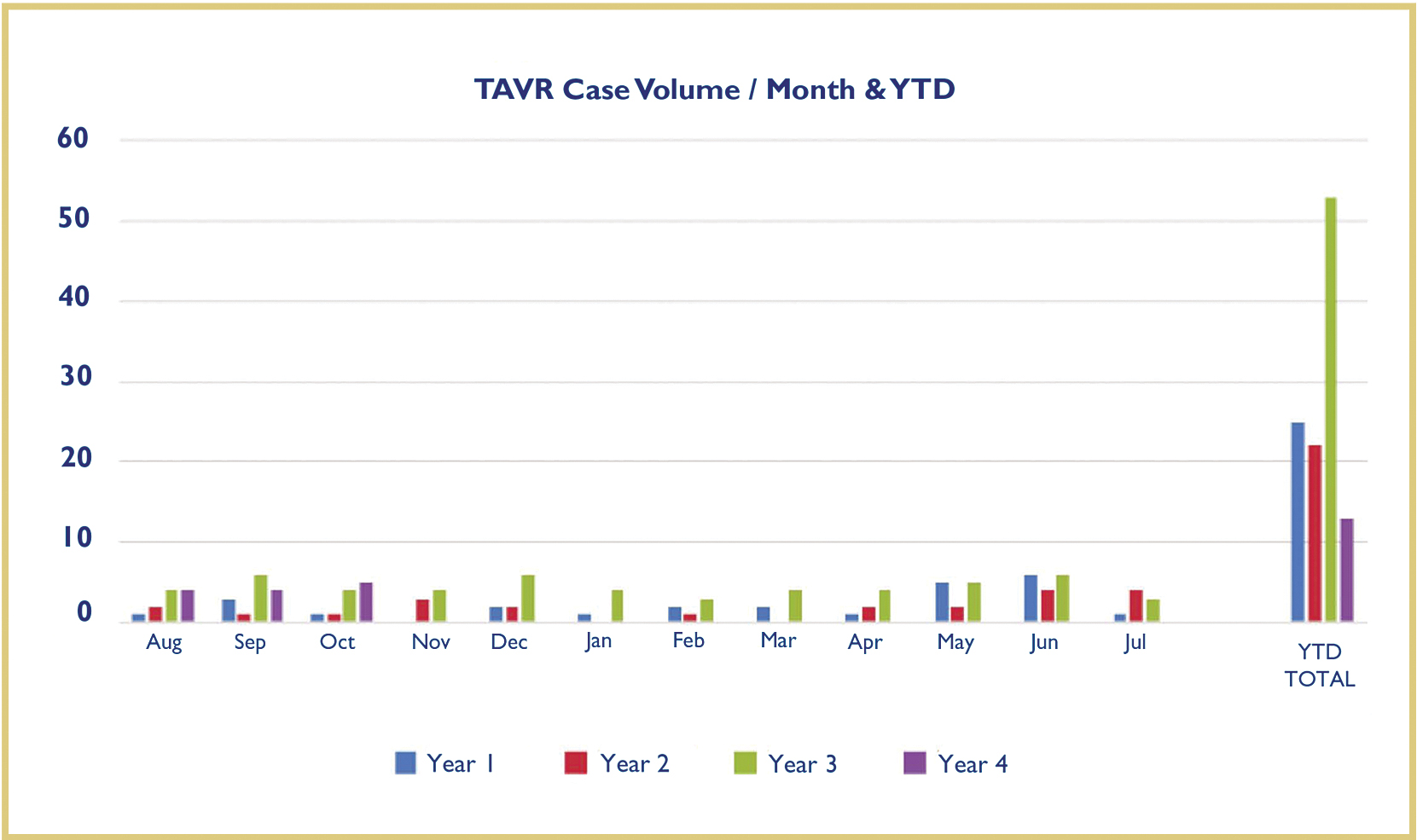
Fig. 6. LGH monthly TAVR volume since August 2012. Blue: Monthly volume from August 2012 – July 2013, Red: Monthly volume from August 2013 – July 2014, Green: Monthly volume from August 2014 – July 2015, Purple: Monthly volume from August 2015 – October 2015. The volume of the program has increased by more than 50% in the last year and continues to increase.
During the first year femoral artery access was used in approximately 50% of cases, but with the newest generation of devices the use of femoral artery access is approaching 80%. Utilization of smaller caliber delivery systems has allowed for the use of conscious sedation rather than general anesthesia. More than 10 patients at LGH have undergone TAVR without the use of general anesthesia or endotracheal intubation. These advancements have resulted in a shorter average length of stay that continues to decrease.
The incidence of moderate aortic insufficiency after TAVR at LGH is 2% and there have been no cases of severe aortic insufficiency. These rates are significantly lower than the reported national average of 5% for moderate or greater aortic insufficiency.21 Two-thirds of patients undergoing TAVR at LGH are discharged directly to home and more than 70% report a large improvement in quality of life. In addition, TAVR outcomes at LGH including mortality rate, stroke, atrial fibrillation, and renal dysfunction have consistently been in the 90th percentile when compared to all other TAVR programs in the United States.
The success of the LGH TAVR program lies in its excellent interdisciplinary collaboration and “team approach.” The TAVR team consists of members from cardiothoracic surgery, interventional cardiology, cardiovascular imaging, congestive heart failure, cardiac anesthesia, critical care and cardiac nursing, physical medicine and rehabilitation, and a dedicated team of specially trained catheterization and surgical nurses and specialists. The TAVR team meets weekly to discuss patient care issues and monthly to discuss program improvement.
CONCLUSION
TAVR has rapidly emerged as a safe and effective alternative for treatment of severe aortic stenosis. It has proven to be superior to medical therapy and at least equivalent to SAVR in patients with a high surgical risk. While several limitations still exist, rapid advancements in this technology are leading to continued improvements in clinical outcomes and decreased complication rates.
The LGH TAVR program has grown rapidly over the past few years and is becoming a high-volume center of excellence. With a focus on collaboration, innovation, and teamwork, the LGH TAVR team is delivering superlative clinical outcomes to the patients of southeast Pennsylvania.
REFERENCES
1. Otto CM, Bonow RO. Valvular Heart Disease. In: Libby P, Bonow RO, Mann DL, Zipes DP, Braunwald E, editors. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 8th ed: Saunders Elsevier, 2008:1625-28.
2. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11.
3. Rosenhek R, Binder T, Porenta G et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med 2000;343:611-7.
4. Carabello BA, Paulus WJ. Aortic stenosis. Lancet 2009;373:956-66.
5. Brown JM, O'Brien SM, Wu C, Sikora JA, Griffith BP, Gammie JS. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg 2009;137:82-90.
6. Bach DS, Siao D, Girard SE, Duvernoy C, McCallister BD, Jr., Gualano SK. Evaluation of patients with severe symptomatic aortic stenosis who do not undergo aortic valve replacement: the potential role of subjectively overestimated operative risk. Circ Cardiovasc Qual Outcomes 2009;2:533-9.
7. Makkar RR, Fontana GP, Jilaihawi H et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med 2012;366:1696-704.
8. Cribier A, Eltchaninoff H, Bash A et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002;106:3006-8.
9. Cribier AG. The Odyssey of TAVR from concept to clinical reality. Tex Heart Inst J 2014;41:125-30.
10. Kodali SK, Williams MR, Smith CR et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012;366:1686-95.
11. Popma JJ, Adams DH, Reardon MJ et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol 2014;63:1972-81.
12. Adams DH, Popma JJ, Reardon MJ et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014;370:1790-8.
13. Leon MB, Smith CR, Mack M et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607.
14. Babaliaros V, Thourani VH, Fearon W et al. 30 day outcomes from the PARTNER II trial for transapical and transfemoral 29mm TAVR in operable patients with the Edwards Sapien XT. J Am Coll Cardiol 2014;63:12_S.
15. Suri RM, Webb JG, Mack M et al. One Year Results of Transcatheter Aortic Valve Therapy for Failed Surgical Bioprostheses- PARTNER II Valve-in-Valve Registry. J Am Coll Cardiol 2014;64:Suppl B.
16. Herrmann HC. SAPIEN 3: Evaluation of a Balloon-Expandable Transcatheter Aortic Valve in High-Risk and Inoperable Patients With Aortic Stenosis – One-Year Outcomes. TCT. San Fransisco, CA, 2015.
17. Manoharan G, Walton AS, Brecker SJ et al. Treatment of Symptomatic Severe Aortic Stenosis With a Novel Resheathable Supra-Annular Self-Expanding Transcatheter Aortic Valve System. JACC Cardiovasc Interv 2015;8:1359-67.
18. Genereux P, Webb JG, Svensson LG et al. Vascular complications after transcatheter aortic valve replacement: insights from the PARTNER (Placement of AoRTic TraNscathetER Valve) trial. J Am Coll Cardiol 2012;60:1043-52.
19. Ussia GP, Barbanti M, Petronio AS et al. Transcatheter aortic valve implantation: 3-year outcomes of self-expanding CoreValve prosthesis. Eur Heart J 2012;33:969-76.
20. Urena M, Webb JG, Tamburino C et al. Permanent pacemaker implantation after transcatheter aortic valve implantation: impact on late clinical outcomes and left ventricular function. Circulation 2014;129:1233-43.
21. STS/ACC Transcather Valve Technologies Registry: Institutional Outcomes Report. 2015.