 Click to Print Adobe PDF
Click to Print Adobe PDF
Fall 2015 - Vol.10, No. 3
|
Are MRIs Safe in Patients with Cardiac Pacemakers and Defribillators?
Sandeep Bansal, M.D., M.P.H.
Electrophysiologist, The Heart Group of Lancaster General Health
Paul Leslie, M.D.
Chairman, Department of Diagnostic Imaging,
Lancaster General Health
Lisa N. Estrella, M.S.
Data Programmer / Statistician,
Lancaster General Research Institute
|
  |
BACKGROUND
Cardiac permanent pacemakers (PPMs) are used in patients with abnormalities of their intrinsic conduction system to prevent slow heart rates. Between 1993 and 2009, their use increased by 50 percent, with 2.9 million PPMs implanted.1 Implantable cardiac defibrillators (ICDs) are used for the prevention of sudden cardiac death due to ventricular arrhythmias. Though initially indicated only for the secondary prevention of sudden death, two landmark trials (MADIT-II and SCD-HeFT) vastly expanded their use to include primary prevention in certain patients with poor cardiac function. As of 2003, an estimated three million people met criteria for insertion of an ICD for either primary or secondary prevention.2 As a result, millions of people in the United States have an implanted cardiac permanent pacemaker (PPM) or defibrillator (ICD), and the number is steadily increasing.
THE PROBLEM
Magnetic resonance imaging (MRI) can provide important imaging information that may not be obtained through computed tomography (CT) scan, X-ray, or ultrasound, particularly with regard to spatial resolution and multi-plane 3-D analysis. MRI is particularly good for soft tissue imaging and is the preferred imaging technique for many neurological and musculoskeletal conditions.3 In addition to its potentially superior imaging characteristics, MRI has the advantage of not exposing patients to ionizing radiation.
PPMs and ICDs have traditionally been considered an absolute contraindication to MRI due to safety concerns regarding the potential interactions between MRI and an implantable cardiac device with metal components. Safety concerns include heating at the lead-tissue interface, mechanical movement of the leads and device in the presence of a strong magnetic field, damage to the electrical circuitry of the device, and device malfunction due to pacemaker resets or pacing changes.4 However, in the face of increasing numbers of implanted cardiac devices, and increasing demand for MRI as the diagnostic modality of choice, it is estimated that up to 75% of patients with a PPM or ICD will need MRI at some point following implantation of their device.2
THE SOLUTION
Device manufacturers have recognized the need to develop MRI conditional cardiac devices, and in recent year, PPMs, ICDs, and leads have been tested and approved, making it possible for patients with these MRI conditional systems to have MRIs. However, less than 1% of current indwelling cardiac devices are MRI conditional.
Since 2001, advances in cardiac devices and MRI technology have decreased the likelihood of device malfunction when exposed to the MRI environment. As a result, several studies have been performed in academic medical centers across the United States to demonstrate the safety of MRIs in patients with PPMs and ICDs. Mollerus and co-workers determined that there was no increase in arrhythmic activity during an MRI scan in patients with implanted devices.3 Nazarian and co-workers also studied patients with PPMs or ICDs who underwent medically indicated MRI, and found that MRI is safe for cardiac device patients when preceded by appropriate screening protocols.5 Their patients experienced no immediate or long-term events that required lead or system revision or reprogramming.6 Similarly, Russo et. al. studied over 800 patients with non-MRI conditional PPMs or ICDs and reported no significant adverse events.7
A STUDY OF MRI FOR CARDIAC DEVICE PATIENTS AT LGH
LGH is the first community hospital in the United States to gain CMS approval for a research study to evaluate the safety and efficacy of MRI in patients with PPMs or ICDs. Lancaster Heart and Vascular Institute is conducting the study, led by principal investigators Sandeep Bansal, M.D., M.P.H., and Paul A. Leslie, M.D.
This is a prospective, single-institution registry of MRI scans in patients with PPMs or ICDs who undergo medically indicated MRIs. The purpose of the registry is to accumulate interrogation data from devices before and after an MRI scan to determine the risk of MRI to patients with implantable devices. The study is similar to studies at Penn Medicine and Johns Hopkins,8 and will allow LGH to gather data for the Centers for Medicare and Medicaid Services to further evaluate the safety of providing access to a wider range of diagnostic imaging modalities in a community hospital setting.
STUDY DESIGN
The registry will acquire and analyze data about the function of devices and the occurrence of adverse events related to the MRI scan. Only patients with implanted devices who have received an order for a clinically indicated MRI scans will be considered for inclusion in the registry. Clinical review will be performed by a designated radiologist to ensure the appropriateness of the MRI request. Any patient with a PPM or ICD system that meets all of the inclusion criteria and none of the exclusion criteria, and who requires a non-cardiac, medically-indicated MRI will be eligible to enroll in the registry. The study’s inclusion and exclusion criteria are listed in Table 1.
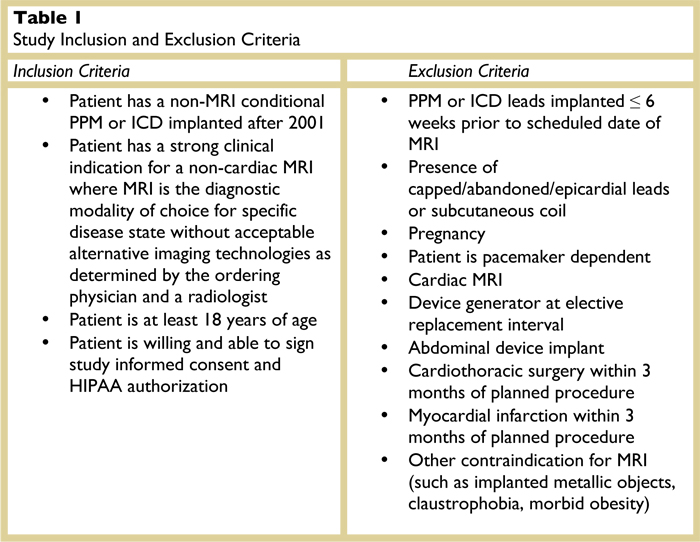
The MRIs will be performed at the LGH Lime Street MRI facility. MRI scan sequences, field intensity, and field(s) of exposure will be selected to minimize risk to the patient while gaining the imaging information necessary for diagnosis or management of therapy.
Study Enrollment
Patients will continue to be enrolled in this registry at LGH until CMS approves the use of MRIs in all patients with non-MRI conditional cardiac devices. We anticipate that at least five-hundred patients will be enrolled. Patients who sign the research consent form and enter the MRI environment will be considered to be enrolled, and will remain so until all post-MRI followup of their device is completed.
Patient Follow-up
Patient follow-up includes a check of the patient’s implantable cardiac device immediately post-scan and again one to six weeks after the scan to confirm appropriate function. Patients with one or more device parameter changes may return for a total of three follow-up device interrogation visits.
The need for device reprogramming will be based on the clinical judgment of the attending electrophysiologist. The results of each follow-up device interrogation will be documented and reported in EPIC device chart and the registry REDCap database (https://redcap.lghealth.org/redcap/).
Reporting
All device-related adverse events/symptoms will be documented on the data collection form and reported to the LGH Institutional Review Board (IRB) and CMS on a quarterly basis. Results will also be updated on the
clinicaltrials.gov website on an annual basis. Registry results will be submitted for publication to one or more peer reviewed journals and may also be presented internally to physicians and other stakeholders who care for the defined study population.
Ordering Physician Workflow
The ordering physician will enter the MRI order and contact the Research Coordinator via the 24- hour Research Hotline (717-902-9544) to provide the patient’s information (name, MRN, reason for MRI, and type of MRI to be done). The Research Coordinator will enroll the patient in the study and coordinate the screening workflow with an electrophysiology specialist, the radiologist and MRI facility, relevant scheduling departments, and the patient.
CONCLUSIONS
By elucidating the safety and efficacy of MRI for patients with PPMs and ICDs, the registry data may impact physicians’ diagnostic choices for this patient population. Patients previously excluded from undergoing MRI despite having conditions for which MRI remains the recommended diagnostic procedure may now be able to do so through participation in the registry. The diagnostic information from the MRI scan may alter treatment decisions and may ultimately affect patient outcomes.
REFERENCES
1. Ferriera, A., Costa, F., et al. MRI-conditional pacemakers: current perspectives. Medical Devices: Evidence and Research, 2014; 7: 115-124.
2. Nazarian, S., Roguin, A., Zviman, M., et. al. Clinical utility and safety of a protocol for noncardiac and cardiac magnetic resonance imaging of patients with permanent pacemakers and implantable-cardioverter defibrillators at 1.5 tesla. Circulation, 2006; 114(12): 1277-1284.
3. Mollerus, M., Albin, G., Lipinski, M., et. al. Ectopy in Patients with Permanent Pacemakers and Implantable Cardioverter-Defibrillators Undergoing MRI Scan. PACE, 2009; 32:772-778.
4. Nordbeck, P., Ertl, G., & Ritter, O. Magnetic resonance imaging safety in pacemaker and implantable cardioverter defibrillator patients: how far have we come? European Heart Journal, 2015; 1:8.
5. Nazarian, S., Hansford, R., Roguiin, A., et.al. A prospective evaluation of a protocol for magnetic resonance imaging of patients with implanted cardiac devices. Annals of Internal Medicine 2011; 155(7), 415-424.
6. Nazarian, S., Beinart, R., & Halperin, H. (2013). Magnetic resonance imaging and implantable devices. Circulation: Arrhythmia and Electrophysiology. 2013;6: 419-428.
7. Magna Safe Registry. Retrieved from http://www.magnasafe.org/aboutmagnasafe.html. November 20, 2014.
8. IRB application for Safety and Clinically Indicated Magnetic Resonance Imaging in Patients with Permanent Pacemakers (PPM) and Implanted Cardioverter Defibrillators (ICDs). Courtesy of Henry Halperin, M.D., M.A. Johns Hopkins Hospital, Baltimore, MD.