 Click to Print Adobe PDF
Click to Print Adobe PDF
Summer 2015 - Vol.10, No.2
|
Mechanical Support of the Circulation: Review and Update on LGH Experience
Mark Epler, MD
Cardiothoracic Surgeon
Jon Echterling, RN, MSN, FNP-BC
LGH LVAD Coordinator
Kelly Trynosky, RN, MSN, ACNP-BC
Lancaster General Health
|
   |
INTRODUCTION
As a result of its high prevalence, morbidity, mortality, and cost of care, heart failure remains a dominant concern of our health care system. It is estimated that 6 million Americans (2.4% of the population) were afflicted with heart failure in 2012.1 This figure is expected to increase by 23% over the next 15 years, so that 1 of every 33 Americans could be diagnosed with heart failure by the year 2030.2
As our population ages, the prevalence of heart failure increases; in individuals 80 years of age or greater its incidence has been reported to be 12%. Direct annual medical costs of heart failure care surpass the $20 billion mark and are projected to nearly triple by 2030. Hospital readmissions for heart failure not only are a major contributor to this staggering cost of care, but also portend limited survival. Whereas a single hospitalization for heart failure among Medicare beneficiaries is associated with a 3-year survival rate of less than 50%, repeated hospitalizations for exacerbations of acute heart failure are associated with a median survival of only 1 year.
Though medical therapy has a major impact on the prognosis and symptoms of early heart failure, it has only a minimal effect on disease progression and survival once patients are hospitalized. Readmissions are frequent, (50% at 6 months) and prognosis remains poor. Until recently, cardiac transplantation provided the only proven and definitive therapy for this population, but the number of donor hearts remains limited. Fortunately, implantation of ventricular assist devices (VAD) has now developed promise as an acceptable alternative that provides definitive therapy for the increasing numbers of patients with heart failure refractory to medical therapy.
INDICATIONS FOR MECHANICAL CIRCULATORY SUPPORT
The traditional indication for implantation of a left ventricular assist device (LVAD) is chronic refractory heart failure in patients who are eligible for and are awaiting cardiac transplantation. This “bridge-to-transplant” classification of LVAD patients was designed to reduce the high mortality in those awaiting transplantation by alleviating their low cardiac output and progressive end-organ deterioration. Two other general categories of VAD patients have since emerged:
a) “Bridge-to-recovery” candidates are afflicted with an insult that results in acute cardiogenic shock, with the majority having had myocardial infarction, fulminant myocarditis, or a complex open heart surgical procedure (postcardiotomy). This category of patients with acute problems requires immediate circulatory support, but since recovery is anticipated, the goal here is stabilization of end-organ function and eventual VAD removal when cardiac recovery permits. The emergence of this category of acute VAD patients expanded the realm of potential candidates beyond those with chronic heart failure. In 2004, we developed the VAD program here at LGH to responsibly allow the performance of high risk open heart surgical procedures within our community. With an implantable ventricular assist device available, the decompensated postcardiotomy cardiogenic shock patient could now be temporarily supported with the hope of myocardial recovery. If they did not recover, they could be evaluated for heart transplantation if appropriate.3
b) In 2001, the landmark REMATCH trial of HF patients ineligible for cardiac transplant showed a 2-fold increase in one year survival and improved quality of life in those who underwent LVAD implantation compared with those who received optimal medical therapy. Thus, “destination therapy” was the second category born out of these data. Subsequently, studies of smaller, more durable, newer generation assist devices have continued to display improved survival over medical therapy in end-stage heart failure patients who require support but have contraindications to transplantation.4
TECHNOLOGY AND CURRENT INDICATIONS FOR LVAD SUPPORT
Currently, two continuous flow devices are FDA approved for use in adult patients.5 The Heartmate II (Thoratec, Pleasanton, CA) continuous flow pump was approved for bridge-to-transplant therapy in 2008 and for destination therapy in 2010. The HeartWare HVAD (HeartWare International, Inc., Framingham, MA) was approved for bridge-to-transplantation therapy in 2012 and is currently being investigated for destination therapy approval.
The Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) is now in its 9th year of data collection, and has compiled data on over 12,000 patients nationwide who have received an FDA-approved durable mechanical circulatory support device since the inception of the registry in June of 2006. In its most recent report, which analyzes more than 10,000 patients who have received a primary implant for left ventricular failure, two dominant trends are highlighted:
First, since their emergence in 2008, continuous flow—rather than pulsatile—pumps continue to dominate the mechanical support landscape. More than 95% of all patients receiving a primary LVAD, and 100% of patients receiving devices for destination therapy, are now implanted with a continuous flow device.
Second, the number of patients receiving mechanical circulatory support as destination therapy continues to grow and overall now represents the largest group of those receiving LVAD devices. In 2006-2007, 14.7% of all LVAD implants were designated as destination care, whereas now the proportion is over 41%. Nationally, 141 hospitals serve as approved DT centers and contribute to the INTERMACS registry. The registry utilizes a patient profile score to stratify a candidate’s pre-implant degree of heart failure and to aid in risk assessment of post-operative death and adverse events. This classification scheme assigns a score of 1 to 7, with the most critically ill patients receiving the lowest scores.
METHODS, INDICATIONS, AND EXPERIENCE AT LGH
In 2010, we published our data on the initial 14 patients at LGH implanted with a mechanical circulatory support device from October, 2005 to December, 2009.3 Survival to hospital discharge was 79% (11/14), with a total of 5 patients ultimately undergoing cardiac transplantation at a regional referral center. First generation, pulsatile heart pump technology dominated this series and was utilized to support patients afflicted with post-operative left ventricular failure and chronic decompensated failure (ischemic or non-ischemic), with the goal of “bridging” these patients to either recovery or transplantation. The final 3 patients in this series were implanted in 2009, and represent our initial foray into continuous flow technology.
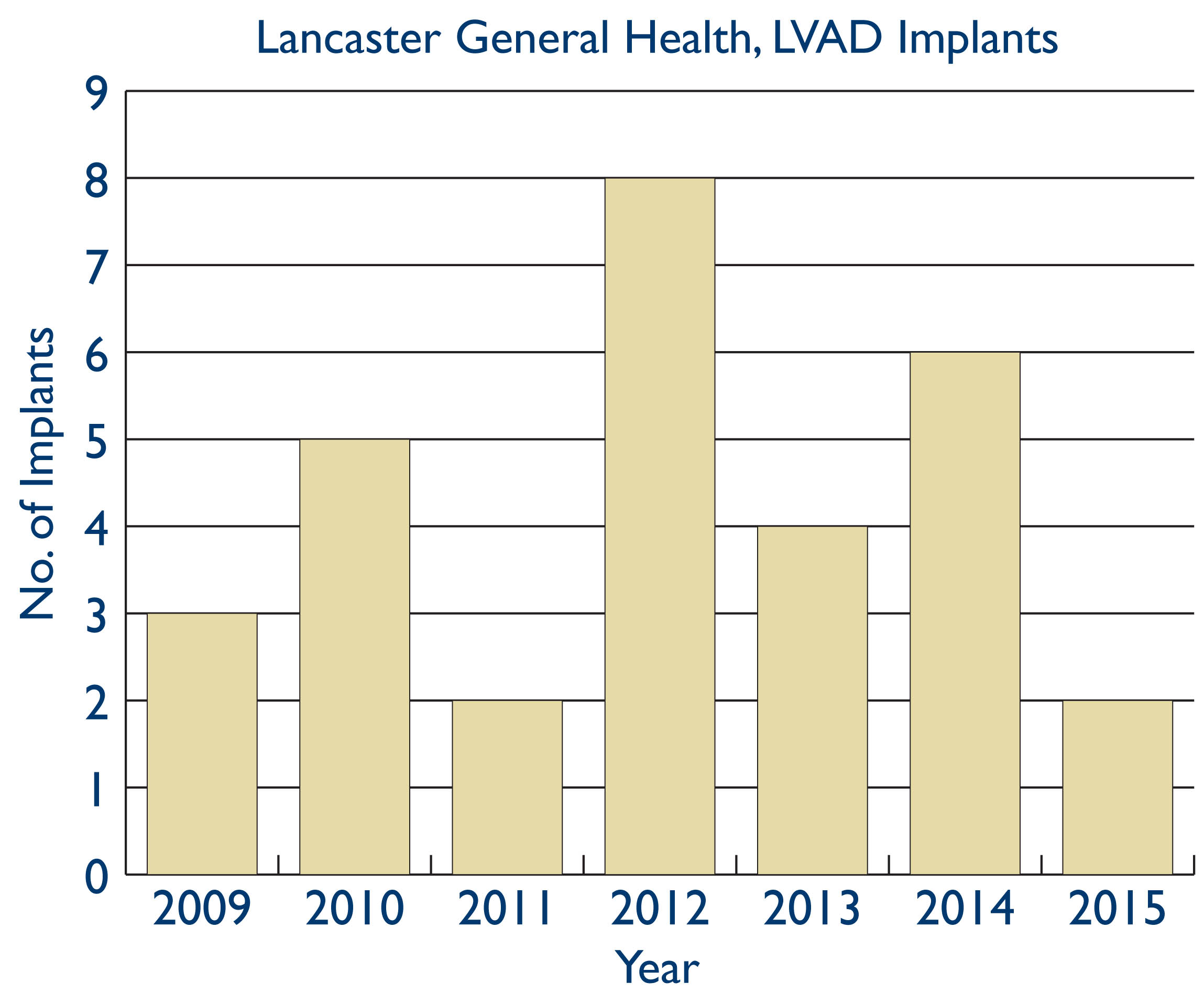
LGH obtained accreditation as a destination therapy (DT) center in 2009. In January, 2010, the FDA approved the Heartmate II continuous flow device for implantation in patients not eligible for heart transplant. Since then, our VAD program has mimicked the nationwide implant trends highlighted above from the INTERMACS Registry. We implanted a total of 30 patients from April, 2009 to April, 2015, of whom 29 received a primary implant with the HeartMate II LVAD. Of these 29, 21 (72%) have been implanted as destination therapy and 8 (28%) were implanted with the goal of eventual heart transplantation.
Demographic data for this cohort of patients implanted with a continuous flow device reveal there were 21 males and 8 females with a mean age of 66 years at implant.
All patients had NYHA Class III or IV symptoms and received inotropic therapy within 48 hours of implantation. 16 of 29 patients (55%) patients were classified as INTERMACS level 1 (critically ill) or 2 (progressive decline despite inotropic therapy) at the time of implant. All patients had LV ejection fractions <30%, and 80% had profound LV dysfunction with EF < 20%. Systolic pulmonary artery pressure was 48 mm/hg with a mean cardiac index of 2.1. Right ventricular (RV) function was severely reduced in only 1 patient; precise pre-implant assessment of RV function remains vital for optimizing outcomes, as post implant RV failure and need for RV mechanical assistance continues to be associated with higher mortality. Average creatinine at the time of implant was 1.45 mg/dl. The 6 patients implanted in 2014 alone accounted for a total of 20 hospitalizations in the year leading up to their implant, with acute heart failure and shock accounting for the vast majority of these admissions.
RESULTS AT LGH
Twenty six of these 29 patients who received a primary continuous flow LVAD between April 2009 and April, 2015 were either discharged from our facility with the device, or were transferred directly to a referral center for subsequent transplant workup. Survival to hospital discharge was thus 90%. One month survival in this series of patients is 93% owing to the death of one patient on post implant day 36 from thrombotic complications related to HITT (heparin-induced thrombocytopenic thrombosis). This patient was never discharged from the hospital following device implant. Eight of 29 patients were implanted as bridge-to-transplant therapy (BTT); 3 of these 8 have been successfully transplanted; 2 are currently supported with their pump; one patient had his device removed 4 years ago and remains alive; one patient recovered adequate cardiac function but expired following device explant; and the final BTT patient died while awaiting transplantation 16 months following device implant. Overall survival in this BTT group is thus 75% (6 of 8), with the longest duration of support now being 6 years.
Survival at 3, 6, and 12 months in the entire series (DT + BTT) is 79%, 70%, and 55% respectively. Though recently implanted patients are not included in survival data because longer followup is needed, the last 6 patients implanted at LGH within the last calendar year are alive. One has gone on to transplant following biventricular support for ischemia-related refractory ventricular tachycardia. The remaining 5 are currently supported as destination therapy.
Overall hospital length of stay has declined from an average of 33 days in 2010 to 24 days in 2014. Post-implant length of stay has also now declined by an average of 3 days over this interval, following an initial increase. Nearly 60% of patients are discharged to home following LVAD implantation, with the remainder having been transferred to either long-term acute care or an in-patient rehabilitation facility. 10% did not survive to hospital discharge.
Of the patients who survived to hospital discharge, 25% have been readmitted within 30 days, an experience that parallels data for all INTERMACS patients implanted over the past 6 years (readmission rate = 27%). The mean pre-implant NYHA functional class in our series is 3.8, and it has improved to 1.3 at one year in all reported surviving patients. This experience is on par with the reported INTERMACS reduction in mean NHYA functional class from 3.8 to 1.8 at one year following successful primary first device implantation.
COMMENT
Most recent INTERMACS data show an actuarial one month survival of 95% for those patients implanted with a continuous flow LVAD from 2008—2013. Our immediate post-implant survival of 90% approaches this benchmark. Of our 2 early post-op deaths, one was attributed to HIT-related complications, and one to acute right ventricular failure. Both of these patients had undergone previous sternotomy (one for AVR, one for coronary bypass), and each required a concomitant valve procedure at the time of their LVAD implant.
Among patients with continuous-flow pumps, actuarial survival per IMTERMACS data continues to be 80% at one year and nearly 70% at 2 years post-implant. In our series, one year survival is 55%. This risk of death following implant appears related to the early phase of our experience, and seems to stabilize near the one year mark, as survival at 2 years in our patients remains above 50%. As our six recently implanted patients pass the one year mark in followup, our overall one year survival statistics will improve markedly. Three patients died of sepsis within the first year following implant; an additional patient succumbed during surgical pleural drainage for a traumatic hemothorax 2 months following implant; and another patient died at the 5 month mark from a massive intracranial hemorrhage. Multi-system organ failure claimed the life of 2 patients within the first year. The need for pump exchange clearly has a negative impact on survival, highlighted by the one year survival of 65% following a second implant. In our one year mortality data reviewed above, pump exchange for device infection or thrombosis was necessary in 2 of the patients who succumbed within one year, and in one patient who succumbed 1.5 years following implant.
Survival in the continuous-flow pump era continues to be negatively impacted by advanced age,6 INTERMACS acuity, progressive renal decline and right heart dysfunction. Registry data continue to display significant hazard ratios (HR) for death in older patients (HR = 1.36); those with INTERMACS profile 1 (HR = 1.69) or 2 (HR = 1.44); those on dialysis (HR = 2.37); those who also receive a right ventricular assist device (HR = 2.45); and those with a history of prior cardiac surgery (HR = 1.43). The need for additional intraoperative procedures at the time of implant (aortic valve replacement, tricuspid valve repair) also negatively impacted survival in our series.
In our series and in nationwide data, perceived quality of life is nearly twice as good as that of pre-implant condition as assessed by standardized health status metrics.
CONCLUSION
The development of continuous-flow ventricular assist devices has produced a significant reduction in adverse events compared with first generation pulsatile heart pumps. Bleeding and infection remain the most common complications occurring within the first year following device implantation, but their frequency has been reduced dramatically and continues to decline with greater experience using continuous-technology. The results of risk stratification for LVAD recipients consistently reveal the worst outcomes in the sickest patients—those designated as INTERMACS profile 1 and 2. These patients not only display poorer survival, but also require much greater postoperative lengths of stay. The majority of implanted patients to date continue to fall into one of these most critical profiles, but as we shift our attention to more destination therapy for heart failure patients, the goal will be to optimize outcomes through patient selection. In recent years, (2011-2014), as part of an effort to improve outcomes and to expand the VAD candidate pool, we are now seeing more patients for implant at less critical stages of their illness, and fewer patients being implanted at INTERMACS level 1 and 2.
Currently, only about 30% of all device implants are performed for patients who are stable on inotropic therapy and less than 20% are performed for “early stage” heart failure patients—those who display limited functional capacity, but have not progressed to inotropic dependence. The patient selection process for VAD implantation is being shifted toward less advanced cases of heart failure by the continued miniaturization of pump design, the evolution of minimally invasive techniques for device implantation, and the reduction in adverse events.7
REFERENCES
1. Kirklin JK, Naftel DC, Pagani, FD, et al. Sixth INTERMACS annual report: A 10,000 patient database. Journal of heart and lung transplantation. 2014; 33: 555-564.
2. Heidenreich, PA et al. Forecastin the Impact of Heart Failure in the United States, Policy Statement From the American Heart Association. Circulation Heart Failure. 2013; 6.
3. Cope JT, Sample S, Small R. Mechanical Cardiac Assist in a Community Hospital Without Transplantation. JLGH. 2010;5:1.
4. Sorabella RA, et al. Comparison of Outcomes After Heart Replacement Therapy in Patients Over 65 years Old. The Annals of Thoracic Surgery. 2015;99;2: 582-588.
5. Ranjit J et. Al. Continuous Flow Left Ventricular Assist Device Outcomes in Commercial Use Compared With the Prior Clinical Trial. The Annals of Thoracic surgery. 2011;92;4: 1406-1413.
6. Williams ML et al. Heart Transplant vs. Left Ventricular Assist Device in Heart Transplant-Eligible Patients. The Annals of Thoracic Surgery. 2011; 91; 5, 1330-1334.
7. Miller LW, Gugline M. Patient Selection for Ventricular Assist Device. JACC. 2013;61(12): 1209-1221.