
Spring 2015 - Vol. 10, No. 1
Update on CT Angiography
Leigh S. Shuman, M.D.
Radiologist
Lancaster Radiology Associates, Ltd.
In the inaugural issue of the JLGH, I wrote about the development of CT angiography (CTA), and ended by urging the reader to “stay tuned,” as it appeared that the use of CTA was destined to explode. It has. In 2006 when that article was published, fewer than 200 cases were done; in 2014, 2,225 CTA’s were performed throughout the institution.
The technology has also continued to advance. A 64-slice CT scanner was considered state-of-the-art back then, and the software needed to process the images and display them was good, but took a good deal of training to use, and at least a few seconds or minutes of processing time to make the images. Our newest scanner at LGH acquires 320 slices in a single rotation of the x-ray tube around the patient, and reconstruction of the images is virtually instantaneous and nearly completely automated.
Starting at the top of the body, CTA has essentially replaced catheter-based angiography for the workup of most conditions involving the head and neck, particularly aneurysms and atherosclerosis. Nearly the only remaining indications for direct catheterization are vasculitis, the detection of which requires extremely high special resolution, and arteriovenous malformations (AVM’s), where the ability to take multiple images over a very short period of time allows the assessment of the inflow and outflow of these unusual lesions.
For carotid and vertebral atherosclerosis, ultrasound is still the initial screening tool, but CTA has largely replaced MR angiography (MRA) as the next step because of it’s superior spatial resolution and much more accurate quantitation of the degree of stenosis. Indeed, the criteria we have always used to measure the degree of stenosis are now seriously out of date. Previously, all stenosis calculations were based on a two dimensional projection of a 3-dimensional vessel, and were expressed as a percentage reduction in luminal diameter. So, for example, a 70% reduction in diameter of a carotid artery was considered significant, and it justified intervention to relieve the obstruction. However, flow is actually determined by luminal area, not diameter, a measurement that could not reliably be made with catheter-based techniques. Now, with CTA, such measurements are routine, as the vessel is scanned from all directions simultaneously and is reconstructed in 3-dimensions. A center-line can be calculated within the vessel, and the images presented as a “curved-planar reformatted” image, so the whole vessel is laid out in a single image, and the cross sectional area precisely orthogonal to the course of the vessel can be seen at any point. (Fig. 1)
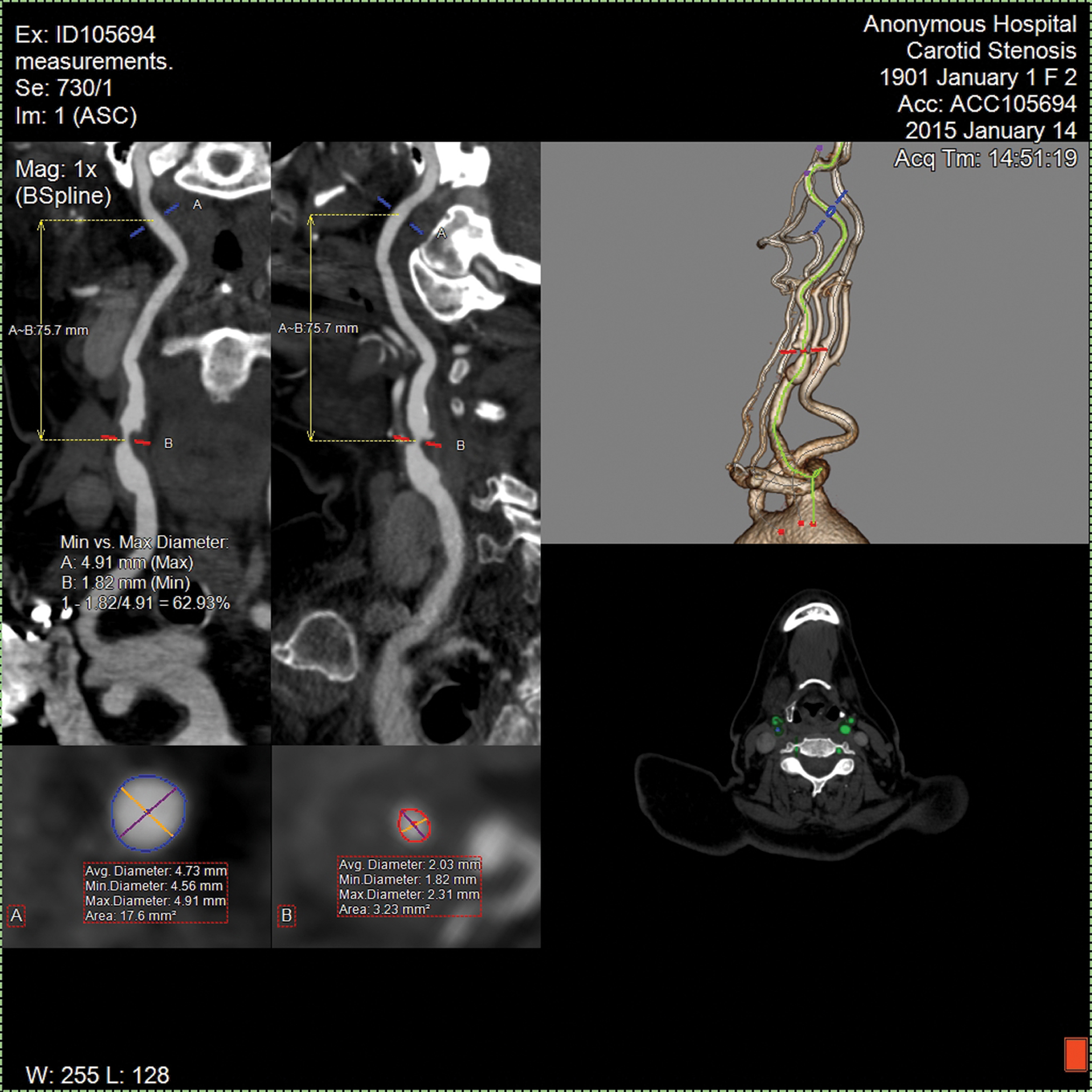
Fig. 1.Evaluation of the carotid artery. The axial image at the bottom right provides the original axial data from which all of the other images are derived; it is actually a stack of very thin axial images covering the entire anatomy of interest. The two images at the upper left represent curved-planar reformatted images of the artery at 90 degrees to each other. The operator can drag the red and blue lines along the image of the vessel, and see the minimum and maximum diameter at any point in the vessel, together with the cross-sectional area displayed in the 2 boxes at the bottom left. The computer will then calculate the percent stenosis based on diameter or area, as desired. This all occurs in nearly real-time once the images are acquired.
In the chest, CTA has become a routine part of the assessment of thoracic aortic disease of all types. With the advent of newer percutaneous techniques for the treatment of aortic valve disease (TAVR procedure) and repair of thoracic aortic aneurysms, CTA is an essential part of the planning process to be sure that these large devices can be delivered safely from the access point, usually the groin, to the site of interest in the chest. Assessment of patients for repeat coronary bypass grafting also often utilizes CTA to make sure no grafts are sitting directly under the sternum, at risk of being damaged when the chest is re-entered.
Stent-graft repair of abdominal aortic aneurysms (AAA) has become routine, and CTA is the tool of choice for planning these procedures, as well as for follow-up to detect leaks and migration of the implanted devices. In addition, the evaluation of a variety of abdominal vascular conditions, including mesenteric ischemia, renal artery stenosis, visceral aneurysms (Fig 2), and even GI bleeding, is done rapidly and non-invasively with CTA, saving the catheterization procedure for the necessary intervention, with a clear plan of how best to perform that intervention.
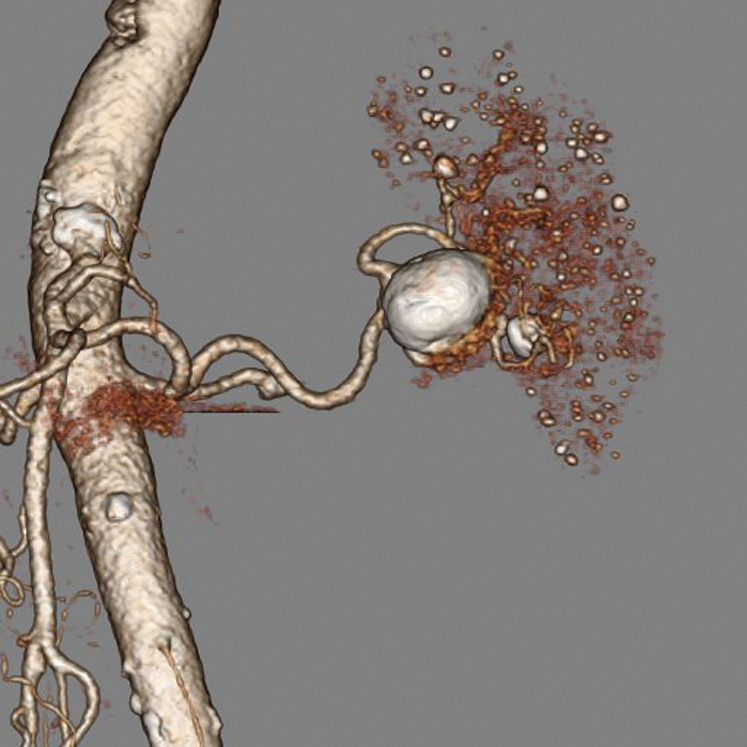
Fig. 2. A volume-rendered image of a splenic artery aneurysm. This image can be rotated in real time and viewed from any angle to see the relationship of the aneurysm to the vessels flowing into and out of it.
This ability to direct catheter-based intervention using CTA is perhaps nowhere better seen than in the extremities, in the diagnosis and treatment of peripheral vascular disease (PAD). Previously, catheter-based angiography was used to first diagnose the location and severity of the blockage(s). Unfortunately, it was not uncommon to discover that the vascular access used to perform the diagnostic study was not the best choice to perform the intervention, or that the appropriate device to treat the lesion was not immediately available. Sometimes it became clear that surgery was the better option once the full picture of the vessels was available. Either a second access, or more likely, a second procedure on a different day was needed to complete the care of the patient. Now, with CTA, the exact extent of disease is known in advance, and the appropriate intervention can be performed much more precisely and rapidly.
The world of trauma care has also benefited greatly from CTA. Previously, if vascular injury was suspected, whether from blunt or penetrating trauma, the angiography team had to be called in and a diagnostic angiogram performed. If active bleeding was found, or a vessel was injured, the injury could then be treated by embolization, stent placement, or surgery. As a result, the time from arrival in the trauma bay to diagnosis of blood vessel injury was measured in hours. Now CTA can easily be added to the routine CT scans being performed to evaluate the trauma patient in the acute phase. Within a few minutes, the presence or absence of active bleeding or vascular injury can be established, and plans for needed treatment can be implemented.
As noted in the original article, there are some downsides to CTA that persist a decade later. Intravenous contrast is still required, and although the amount needed has decreased somewhat, the procedure is still relatively contraindicated in the setting of renal insufficiency or major allergy. Concern for radiation dose has also increased in the past decade, in some patients almost to the point of hysteria. While CTA often involves the same or more radiation than a conventional angiogram, the manufacturers of CT equipment have made major strides in reducing the dose. Given the quality of diagnostic information obtained the risk is extremely small, as well as posing a risk that is much lower than the risk of complications from a diagnostic catheterization. Though extremely dense calcification can present difficulty in obtaining precise measurements, that doesn’t negate the value of the procedure.
It is tempting to say that we have gone about as far as we can go with CTA, but given the rapid growth in the past decade, there will undoubtedly be significant improvements in speed, accuracy and safety in the next 10 years. Once again, stay tuned.