|
Abstract
Chronic kidney disease (CKD) is a complex syndrome with a variety of comorbidities; in patients on hemodialysis, the most common are cardiovascular disease (CVD) and metabolic bone disease (renal osteodystrophy).1 The pathogenesis of renal osteodystrophy involves abnormal vitamin D levels and secondary hyperparathyroidism (SHPT); the latter may also play a role in the pathogenesis of CVD. Furthermore, recent studies suggest a link between bone disease and vascular calcification, which also increases the risk of cardiovascular complications.
Since abnormalities in vitamin D levels and SHPT can be reversed by vitamin D supplements in patients with early CKD, early intervention may reduce pathological changes in bone structure and metabolism, vascular calcification, and the risk of cardiovascular events.
Vitamin D Metabolism & Secondary Hyperparathyroidism (SHPT)
As detailed elsewhere in this issue of JLGH,2 Vitamin D is synthesized in the skin in response to ultraviolet radiation, and is also derived from multiple fortified foods such as milk and cereals, fatty fish, fish liver oils, and egg yolks. The vitamin’s main function is to maintain calcium and phosphate homeostasis, and it therefore has a key role in normal bone mineralization, growth, and repair. There are two main forms of vitamin D in the body: 25(OH) vitamin D, a partially activated form produced in the liver, and 1,25 dihydroxycholecalciferol (OH) vitamin D, the fully active form. The kidneys are the primary sites of 1-hydroxylation (addition of an OH group).
The production of 1,25(OH) vitamin D is impaired in CKD patients. Insufficient levels of fully activated vitamin D become evident in CKD stages 2 to 3 (glomerular filtration rate [GFR] near 50cc/min) as the volume of functioning kidney mass decreases. The deficiency stimulates the parathyroid glands to increase the secretion of parathyroid hormone, and elevated parathyroid hormone levels may be evident long before abnormalities in calcium and phosphate levels become clinically apparent (Figure 1). This maladaptive process of SHPT can lead to progressive nodular, adenomatous-like changes in the parathyroid gland.
Bone Disease in CKD
Renal osteodystrophy, a consequence of vitamin D and parathyroid hormone abnormalities in patients with CKD, takes one of two forms: high-turnover syndromes typified by enhanced bone resorption (osteitis fibrosa), and low-turnover syndromes typified by impaired mineralization (adynamic bone disease). These varieties of disease manifestations are influenced by patient age, genetics, underlying cause, and duration of CKD, disease severity, diet, interventions, dialysis use and duration, aluminum burden, and diabetes. Patients typically complain of nonspecific bone pain, weakness, and skeletal deformities, and fractures are common.
|
Figure 1: Progression of 1,25-dihydroxyvitamin D deficiency and hyperparathyroidism among patients with CKD stages 1–4.
|
|
|
Source: Andress DL. Vitamin D in chronic kidney disease: A systemic role
for selective vitamin D receptor activation. Kidney Int. 2006;69: 33–43.
Reprinted by permission from Macmillan Publishers Ltd. |
In patients with stage 3 or 4 CKD, high-turnover bone disease—osteitis fibrosa—is the most common type, affecting up to 75% of patients in these early stages, according to a 1995 study (Figure 2).5 Osteitis fibrosa is characterized by elevated parathyroid hormone levels, and normal, or near normal, calcium levels. As parathyroid hormone levels increase, bone mineral density may decrease. In contrast, low-turnover bone diseases are uncommon in patients with stage 3 or 4 CKD, but they predominate in stage 5 patients.
|
Figure 2: Incidence of metabolic bone disease in CKD stages 3 and 4.
|
|
|
Source: Hamdy. Effect of alfacalcidol on natural course of renal bone
disease in mild to moderate renal failure. BMJ. 1995;310:358-363. Permission granted, copyright 2004, The Endocrine Society. |
Bone Disease and Cardiovascular Disease
Evidence that soft tissue calcification and, in particular, cardiovascular calcification, is a consequence of bone disease, comes from several sources. In a recent study of 2,348 postmenopausal women, as bone density decreased aortic calcification increased proportionally over the 9-month study period (Figure 3).6
The presence of arterial calcification in CKD patients has been documented in numerous other studies. Kramer and colleagues reported substantially higher coronary artery calcification scores in patients with CKD stages 3 to 5 (GFR <60 cc/min) than in those in earlier stages.7 Calcification scores were notably increased among those with concomitant diabetes. Valvular and arterial calcifications are particularly prevalent in hemodialysis patients,8 and tend to occur at younger ages than in the general population, sometimes even in children.9
|
Figure 3: In postmenopausal osteoporosis, aortic calcification increases proportionally as bone mineral density decreases.
|
|
|
Source: Schultz E, et. al. Aortic calcifi cation and the risk of osteoporosis
and fractures. J Clin Endocrinol Metab. 2004;89:4246-4253. Permission
granted, copyright 2004, The Endocrine Society. |
The increased arterial calcification in CKD has been identified as a potential mediator of CVD and an indicator of increased mortality risk. Arterial calcification in CKD is associated with an increased prevalence of coronary artery disease, peripheral vascular disease, left ventricular hypertrophy, and death, perhaps because arterial calcification increases arterial stiffness, which may raise pulse pressure, damage left ventricular function and incite hypertrophy (leading to heart failure).10 Increased arterial stiffness may also increase the risk of plaque rupture and thrombosis.
Soft tissue calcification in dialysis patients has also been attributed to overall positive calcium balance generated by diet, calcium-containing binders, and dialysis baths with vitamin D and calcium levels equivalent to serum calcium concentrations of 10mg% or more. Hyperphosphatemia, which can contribute to a high calcium-phosphorous product and presumed increased calcification, has been shown to be a significant, independent factor contributing to mortality in dialysis patients.11 In vitro, elevated phosphorous levels have been shown to induce bone protein gene transcription in vascular smooth muscle cells.12
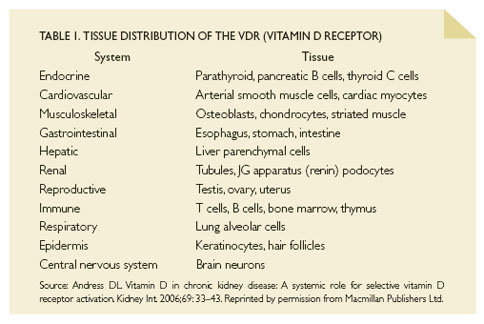
Vitamin D receptors may provide another pathway to vascular injury, as they are ubiquitous throughout the body, and have a role in regulating multiple genes other than those related to calcium and bone metabolism (Table 1). For example, current research indicates that vitamin D may suppress the renin-angiotensin-aldosterone system, T cells, and other mediators of inflammation, and that it may have independent effects on left ventricular hypertrophy, atherosclerosis, and immune responses. Future studies are needed to further address the contribution of selective vitamin D receptor activation in health and disease.12
Vitamin D Mortality Studies
At least one large observational study associated vitamin D repletion with reduced mortality among dialysis patients.11 Teng and colleagues followed 51,037 patients who survived for at least 90 days following the initiation of dialysis for 2 years. Of these, 37,173 received some form of injectable vitamin D and 13,864 received no vitamin D. Two-year survival was 75.6% among vitamin D-treated patients versus 58.7% among those who received no treatment (p<0.001). After adjustment for potential confounders such as age, gender, race, and diabetes, among others, mortality continued to be 20% lower with any form of injectable vitamin D repletion. Vitamin D therapy also significantly lowered cardiovascular-related mortality (7.6 per 100 person-years versus 14.6 per 100 person-years, p<0.001). Treatment was beneficial in 48 of the 49 subgroups studied, including patients with low serum intact parathyroid hormone levels, and those with hypercalcemia who usually do not receive vitamin D therapy.
Treatment Options in Early CKD
Addressing the underlying causes of renal osteodystrophy—vitamin D deficiency and SHPT—during the early and middle stages of CKD may help slow the progression of bone disease and prevent arterial calcification.
Testing—Guidelines from the Kidney Disease Outcomes Quality Initiative (K/DOQI) recommend routine testing of serum calcium and phosphorus levels, and plasma parathyroid hormone levels, in all patients with a GFR <60 cc/min.12 This is a new paradigm, as previously parathyroid hormone levels were only measured if calcium and phosphorous abnormalities were clinically apparent. Parathyroid hormone levels are now measured annually in stage 3 patients, and every 3 months in stage 4 patients. More frequent parathyroid hormone testing is recommended for patients receiving vitamin D treatment, along with routine monitoring of calcium and phosphorus levels. Intact parathyroid hormone levels < 70 pg/mL in stage 3 patients, and < 110 pg/mL in stage 4 patients, are considered acceptable.
Calcium and phosphorous need to be monitored closely during vitamin D therapy. Hypercalcemia (calcium-phosphorous product >55 mg2/dL2), is abnormal, and evidence of a positive calcium balance—a risk of activated vitamin D therapy.12 Ergocalciferol (a non-activated vitamin D formulation) does not cause hypervitaminosis D unless doses exceed 2,400 IU per day.
Dietary restrictions—Dietary phosphorous restriction (1,000 mg/day) may be implemented in stages 3 and 4 when hyperphosphatemia is present. If dietary measures do not decrease phosphorus, phosphate binders may be used. Calcium-based phosphate binders (carbonate or acetate) are effective; however, careful monitoring for hypercalcemia is required in stage 3 and 4 patients, and there is ongoing debate regarding the potential risks of calcium loading with these agents in stage 5 patients. Sevelamer, a non-calcium resin binder, is effective, and is not associated with the potentially hazardous calcium loading seen with calcium-based agents. However, it is expensive, and may worsen metabolic acidosis. Lanthanum carbonate, a newer, non-calcium binder is also expensive, and has some long-term safety issues (e.g., liver toxicity and bone accumulation).11
Vitamin D supplementation—Vitamin D repletion in stages 3 and 4 is warranted when serum 25(OH)D levels are <30 ng/mL and parathyroid hormone levels are above the target range for the CKD stage. K/DOQI recommends vitamin D supplements of 50,000 units given weekly or monthly depending on the severity of the deficiency. Multiple activated vitamin D formulations (calcitriol, doxercalciferol, and paricalcitol) are available, and all have been shown to reduce parathyroid hormone levels, but with differing safety profiles (i.e., risk of hypercalcemia or calcium loading).8
Some data suggest that paricalcitol is the preferable agent, but randomized comparisons are lacking. K/DOQI guidelines recommend monthly testing of serum calcium and phosphorus for the first 3 months of treatment with this agent, and every 3 months thereafter.
Conclusions
Vitamin D deficiency leading to SHPT and renal bone disease is a common and potentially hazardous complication of CKD. Abnormal bone mineralization is associated with soft tissue, and, in particular, arterial calcification, which may increase the risk of cardiovascular-related morbidity and mortality. Vitamin D therapy may suppress SHPT and retard the progression of renal osteodystrophy. Careful monitoring of vitamin D and parathyroid hormone levels may help prevent bone and vascular changes, and reduce mortality.
References
- Carroll L. The Stages of Chronic Kidney Disease and the Estimated Glomerular Filtration Rate. J Lancaster Gen Hosp. 2006; 1: 64-69. [Vol. 2, Fall])
- Reese RW. Vitamin D and Bone Health. Jl Lancaster Gen Hosp. 2006; Vol 1, No 3:78-87
- Hamdy NA, Kanis JA, Beneton M, et al. Effect of alfacalcidol on natural course of renal bone disease in mild to moderate renal failure. BMJ. 1995;310:358-363.
- Schultz E, Arfai K, Liu X, Sayre J, Gilzanz V. Aortic calcification and the risk of osteoporosis and fractures. J Clin Endocrinol Metab. 2004;89:4246-4253.
- Kramer H, Toto R, Peshock R, Cooper R, Victor R. Association between chronic kidney disease and coronary artery calcification: The Dallas Heart Study. J Am Soc Nephrol. 2005;16:507-513.
- Strozecki P, Odrawaz-Sypniewska G, Manitius J. Cardiac valve calcification and left ventricular hypertrophy in hemodialysis patients. Ren Fail. 2005;27:733-738.
- Salusky IB, Goodman WG. Cardiovascular calcification in end-stage renal disease. Nephrol Dial Transplant. 2002;17:336-339.
- Andress DL. Vitamin D in chronic kidney disease: A systemic role for selective vitamin D receptor activation. Kidney Int. 2006;69:33-43.
- Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phospate product with mortality risk in chronic hemodialysis patients: A national study. Am J Kidney Dis. 1998;31:607-617.
- Giachelli CM. Vascular calcification mechanisms. J Am Soc Nephrol. 2004;15:2959-2964.
- Teng M, Wolf M, Ofsthun N, et al. Activated injectable vitamin D and hemodialysis survival: A historical cohort study. J Am Soc Nephrol. 2006;16:1115-1125.
- National Kidney Foundation. K/DOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease. Available at: http://www.kidney.org/professionals/kdoqi/guidelines_bone/index.htm. Accessed September 12, 2006.
- Friedman EA. Calcium-based phosphate binders are appropriate in chronic renal failure. Clin J Am Soc Nephrol. 2006;1:704-709.
Laurence E. Carroll, M.D., F.A.S.N.
Hypertension Kidney Specialists
2112 Harrisburg Pike - Suite 312
Lancaster, PA 17604
717-544-3232
lecjtc@comcast.net
|