 Click to Print Adobe PDF
Click to Print Adobe PDF
Spring 2006 - Vol.1, No.1
|
BENEFITS OF VENTRICULAR ASSIST DEVICES
FOR HIGH-RISK CARDIAC PATIENTS
IN A NON-TRANSPLANT SETTING
Edward F. Lundy, M.D., PH.D.
Director, VAD Program, Lancaster General Hospital
Cardiothoracic Surgeons of Lancaster, P.C.
Susan Sample, MSN, CRNP
Program Manager, Cardiothoracic Surgery,
Lancaster General Hospital
|
|
Abstract
Ventricular assist devices have historically been used as a bridge to transplantation, and most of the early clinical trials focused on this indication. Recently, the indications have expanded to include both support to recovery and destination therapy. The clinical indications for VAD therapy can include one or more of the following: post cardiotomy failure, acute myocardial infarction, acute decompensated heart failure, myocarditis, ventricular arrhythmias and high risk cardiac operations. Lancaster General is one of only 3 non-transplant centers in the nation to use the technology as part of a comprehensive treatment approach for severe congestive heart failure (CHF) patients. VADs can provide a safe and effective treatment for many of these disease states as either a short-term or a long-term therapy option.
From September 2004 until January 2006, we evaluated 20 high risk surgery candidates for possible use of a VAD. The majority of patients were treated with advanced surgical therapies, including coronary artery bypass grafting, mitral valve repair, and Dor procedures. Two patients received VADs, which saved their lives. The first patient presented with an acute myocardial infarction complicated by cardiogenic shock and would not have survived without immediate, emergency surgery. The second patient had undergone coronary artery bypass graft surgery, however the onset of lethal arrhythmias 12 hours postoperatively demanded emergency surgery requiring a biventricular device. In both cases, the patients are alive and well today thanks to the availability of VADs at Lancaster General Hospital.
We plan to continue to offer VAD therapy as a back-up method of stabilizing patients who undergo high risk surgery. These patients may either require heart transplantation or may be mechanically supported as an alternate treatment for those ineligible for transplant. We believe these device-based approaches are an integral part of any high-risk cardiovascular surgery program and will play an increasingly important role in treatment for the growing number of patients with congestive heart failure.
Introduction
The prevalence of congestive heart failure diagnosis is growing at an alarming rate. More than 5 million people in the United States have been diagnosed with congestive heart failure. Approximately 550,000 new cases are diagnosed every year, and 57,000 heart failure deaths occur annually. Further, while the overall death rate in the US population declined two percent from 1993-2003, deaths from heart failure increased 20.5 percent.1 We can expect that figure to continue rising: a recent study funded by the National Heart, Lung and Blood Institute found that more than half of men and nearly 40 percent of women will develop cardiovascular disease in their lifetime.2 The financial burden associated with this disease is calculated at $27.9 billion in 2005, and will continue to increase over time.
Despite the increased attention given to cardiac risk factors such as obesity, cholesterol, high blood pressure and diabetes, heart disease remains the number one killer in America, due to a number of societal and economic factors. Cholesterol levels are just one example: less than half of the people at the highest risk for symptomatic coronary heart disease receive lipid-lowering treatment, based on data from the Third Report of the Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. A mere 10 percent decrease in Americans’ total cholesterol levels would result in an estimated 30 percent reduction in coronary heart disease.3
As the nation’s waistline expands and blood pressures rises, physicians and surgeons are continually asked to find new and innovative cardiac treatments and procedures that push the envelope. The increase in the frequency of these procedures is astounding — cardiac catheterizations rose 373 percent from 1979-2003 and percutaneous coronary interventional procedures increased 326 percent from 1987-2003.1 But hearts can only be repaired so often, and patients who received stents and angioplasties in their 40s or 50s, may find themselves facing congestive heart failure twenty years later.
For these patients, heart transplantation may be the best course of treatment — but only if they are fortunate enough to be eligible for transplantation and then actually receive a new heart. In any given year, as many as 100,000 patients are eligible to receive a heart transplant but only about 2,200 donor hearts are available.
4 More than a decade ago, researchers began early trials of ventricular assist devices (VADs) to offer these remaining patients an alternative, either as a temporizing bridge to transplantation, or as “destination therapy.” The latter term means that when a VAD is implanted, the patient lives with the device until the end of their life. Destination therapy was embraced by the medical community based on the success of the REMATCH trial (Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure). That study compared patients who received VADs with those who received standard medical therapy, and found that VADs significantly improved survival rates in patients who were not transplant candidates.
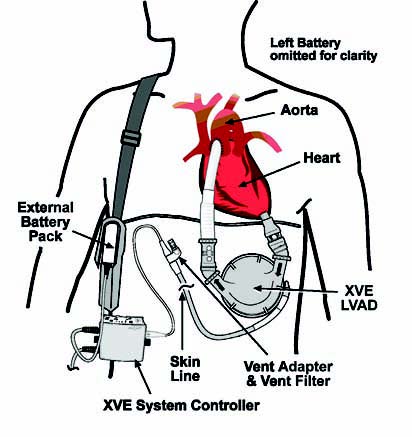
Figure 1: HeartMate® XVE LVAS
After REMATCH demonstrated the potential of VADs, in 2003 the FDA approved the use of one brand of VAD — Thoratec’s HeartMate XVE — as destination therapy for patients with New York Heart Association Class IV heart failure who are ineligible for transplant.
5 Most recently, as the technology and understanding of these devices has improved, morbidity rates have dropped and the technology has been increasingly considered as a bridge to transplantation or as an alternative to transplant. A 2005 study published by the University of Pittsburgh found VADs to be a justifiable strategy for patients presenting with morbid congestive heart failure. In particular, researchers noted that morbidity associated with VADs has been significantly reduced in the past four years, and management as an outpatient is achievable.
6 Columbia University researchers report similar conclusions, anticipating that device-based approaches will assume an increasingly important role in treating advanced heart failure.
7
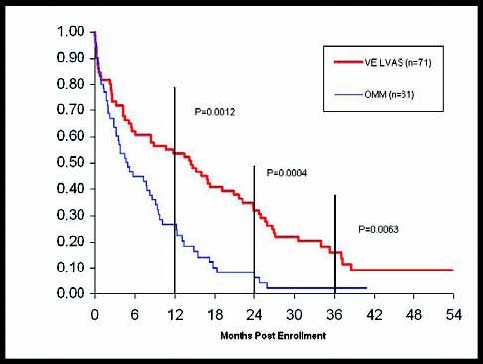
Figure 2: REMATCH Update (as of April 2004) – Source Thoratec Registry
Clinical Experience
All the above reports lead to the conclusion that if non-transplant centers in community-based hospitals intend to provide complete congestive heart failure programs, they should have VADs available, either as a bridge to transplantation, bridge to recovery (for patients after cardiac surgery), or destination therapy. That is our belief at Lancaster General Hospital, and is one of the many reasons we now offer VADs as part of our comprehensive congestive heart failure care. From September 2004 through January 2006, we considered 20 patients for VADs, 4 of whom did not have any surgical procedure because they refused surgery or were not adequate surgical candidates, and 16 of whom underwent high risk cardiac surgery.
These devices literally saved the lives of 2 of those 4 surgical patients. The first was a 41-year-old man brought to the ER with acute chest pain that was decompensating rapidly.
Figure 3: Thoratec¨ VAD Illustration – Thoratec Corporation
He had sustained a massive myocardial infarction less than 6 hours before arriving at the ER and had no chance of survival without immediate surgery. Since both ventricles needed support, both a left and right VAD were implanted. After he was stabilized, he was transferred to the Hospital of the University of Pennsylvania where he received a heart transplant approximately three months later. He is currently at home and successfully recovering following the transplantation.
The second patient, a 47-year-old man, entered the ER with chest pain and we determined that he had sustained a myocardial infarction more than 21 days previously. We carried out an elective coronary artery bypass graft (CABG) procedure, but on the first postoperative day he decompensated and required treatment for lethal arrhythmias. Left and right VADs were implanted and,once stabilized, he was also transferred to the Hospital of the University of Pennsylvania for heart transplantation, which was performed one month later. He is now doing well.
Figure 4: Thoratec¨ Percutaneous VAD System – Thoratec Corporation
Bridge To Transplantation
The use of a ventricular assist device as a means to bridge a patient to heart transplantation is well studied. The absolute indications for heart transplant include: refractory cardiogenic shock, dependence on inotropic drugs, persistent heart failure symptoms at rest, and refractory arrhythmias. The above mentioned mismatch between the number of patients requiring heart transplant and the number of available donor organs8 means that ventricular assist devices give patients with end-stage heart failure the opportunity to wait for availability of a transplant with the added benefit of reversal of end-organ dysfunction. Research has shown that promptness of implantation of ventricular assist devices, as well as early device removal and transplantation, is associated with improved survival post-transplantation.9
Bridge To Recovery
It is not uncommon for VADs to be used as a back-up procedure in cases where additional complications prevent the success of high-risk, open-heart surgery. As a leading non-transplant cardiovascular surgery center, with particular success in high-risk patients, we felt that we had to add this technology to our services. Since acquiring VAD capability, we have considered 16 surgical candidates for a VAD, of whom 12 presented with congestive heart failure. These high-risk patients were all successfully treated: the two described above received VADs, 10 others received CABGs, one received a CABG with a mitral valve repair, and one received a CABG in combination with a mitral valve repair and a Dor procedure (surgical ventricular remodeling). Of the four patients who were not in heart failure, two received CABGs, one received a CABG re-do, and one received a CABG in combination with a mitral valve repair and radiofrequency ablation. The vast majority of patients (13 out of 16) were 58 years or older, and half of the patients required minimal hospitalizations of 14 days or less.
We review a number of risk factors present in high-risk cardiovascular patients to determine the best course of treatment. Among them are diabetes, dyslipidemia, renal failure, hypertension, previous cardiovascular intervention, myocardial infarction, congestive heart failure, angina, cardiogenic shock, arrhythmia, and ejection fraction. Of the 16 high-risk patients successfully treated at Lancaster General Hospital, 5 had diabetes, 14 had dyslipidemia, 3 had renal failure, 11 had hypertension, 9 had angina (5 unstable), one was in cardiogenic shock, and 15 had ejection fractions less than 39 percent preoperatively.
Destination Therapy
The term “destination therapy” is relatively new in the last 5 years, and provides an alternative to transplant in patients with end stage heart failure (ESHF). With the current innovative pharmaceutical, device, and surgical techniques available, ESHF patients are living longer. This phenomenon, combined with the rising age of the general U.S. population, constantly challenges the medical community to provide other options that improve morbidity and mortality in patients with ESHF, and makes destination therapy an inevitable future direction for the majority of comprehensive heart failure programs. Since heart transplantation is not able to meet the growing needs of the ESHF population, VADs may be able to pave the way to a longer and better quality life. This treatment option will be considered at our hospital as we evolve and further develop our heart failure program.
Discussion
A conventional cardiac surgical procedure such as CABG or valve surgery is always preferable to a VAD implant or heart transplantation, but despite their long-term benefits, these primary surgical procedures are not immediately beneficial to all high risk patients. By having the option of VAD therapy, Lancaster General Hospital has been able to offer a higher level of surgical options to even our sickest patients. The 16 high-risk patients followed here have all recovered or are on their way to recovery, and we expect them to do very well in the long-term. The availability of the VAD has complemented our existing services and expanded their capability. The two patients who entered the ER with acute heart failure simply would not have survived had it not been for the devices. Not only are these patients alive today, but their quality of life while awaiting a donor heart was greatly improved.
Among non-transplant centers, Lancaster General offers one of the most comprehensive CHF programs in the nation. But though we are a leading community hospital in terms of volume of CHF patients treated, we do not perform heart transplantation and do not intend to. Since only about 2,200 heart transplantations are performed each year in the U.S., it’s imperative that transplantation be isolated to high-volume transplant centers, where success can be more readily assured. However, the high volume of non-transplant open-heart operations performed at Lancaster General, combined with our expertise with new and ever-advancing technologies (see the article by Dr. Roy Small in this issue of the Journal), enables us to successfully treat the growing number of CHF cases. Further, by offering VADs, and partnering for transplants with the Hospital of the University of Pennsylvania, we’re offering patients and transplant surgeons the one thing that often eludes them — time. The overall goal of our program is to maintain our high quality of care, and to improve the quality and length of life of our heart failure patients.
Referecnes
1. American Heart Association. Heart Disease and Stroke Statistics — 2006 Update. Dallas, Texas: American Heart Association; 2006.
2. Leip E, Larson, M, D’Agostino, R, Beiser A, Wilson P, Wolf P, Levy D. Gazing at the crystal ball: researchers calculate lifetime risk of CVD. Circulation: Journal Report, February 7, 2006. http://www.heart.org/presenter.jhtml?identifier=3037324.
3. State-Specific Cholesterol Screening Trends — United States, 1991-1999. MMWR. 2000; 49 (33): 750-755.
4. Stevenson L, Rose E. Left Ventricular Assist Devices, Bridges to Transplantation, Recovery and Destination for Whom? Circulation. 2003; 108 (25): 3059-3063.
5. Rose, E, Geligjns, A, Moskowitz, A, Heitjan, D, Stevenson, L, Dembitsky, W, Long, J, Ascheim, D. Tierney, A, Levitan, R, Watson, J, Ronana, N, Meier, P. Long-Term Use of a Left Ventricular Assist Device for End-Stage Heart Failure. The New England Journal of Medicine. 2001. 345(20): 1435-1443.)
6. Tsuki H, Teuteberg J, Murali S, McNamara D, Buchanan J, Winowich S, Stanford E, Mathier M, Cadaret L, Kormos R. Biventricular Assist Device Utilization for Patients with Morbid Congestive Heart Failure. Circulation. 2005; 112: I-65 – I-72.
7. Mancini D, Burkhoff D. Mechanical Device-Based Methods of Managing and Treating Heart Failure. Circulation. 2005; 112 (3): 438-448.
8. Stevenson L, Rose E. Left Ventricular Assist Devices, Bridges to Transplantation, Recovery and Destination for Whom? Circulation. 2003; 108 (25): 3059-3063.
9. Gammie J, Edwards L, Griffith B, Pierson R, Tsao L. Optimal timing of cardiac transplantation after ventricular assist device transplantation. J Thorac Cardiovasc Surg. 2004; 127(6): 1789-99.
Edward F. Lundy, M.D., Ph.D.
Director, VAD Program, Lancaster General Hospital
Cardiothoracic Surgeons of Lancaster, P.C.
555 N. Duke Street
Lancaster, PA 17604
Susan Sample, MSN, CRNP
Program Manager, Cardiothoracic Surgery,
Lancaster General Hospital
555 N. Duke Street
Lancaster, PA 17604
717-544-4847