|
|
|

Spring 2011 - Vol.6, No.1
Oligodendroglioma: A Riddle Inside a Misnomer
Wrapped in an Enigma
The Oligoemperor Has No Clothes!
Charles Romberger, M.D.
Pathologist
INTRODUCTION
Glial cells are non-neuronal cells in the central nervous system that surround, protect, and sustain the neurons The two most numerous normal glial cell types in the CNS are the astrocyte and the oligodendrocyte. During embryologic development, the neuropithelial lining cells first produce neuroblasts, the progenitors of neurons. As soon as neuroblast production ceases, this same neuroepithelial lining begins to produce glioblasts, which differentiate into astrocytes and oligodendrocytes.7 Recent studies suggest that both oligodendrocytes and at least some astrocytes are derived from the same progenitor cells.1,3,16 Astrocytes normally serve a sustentacular function, i.e. they support and sustain neurons. Oligodendrocytes normally serve an insulating function, i.e. they serve to myelinate within the central nervous system. In sum, both oligodendrocytes and type 2 astrocytes are derived from a common precursor, and, despite their very different functions, they have a close embryologic relationship.
Excluding ependymal tumors and mixed neuronal-glial tumors, the World Health Organization essentially recognizes two types of glial tumors: “Astrocytic tumours” and “Oligodendroglial tumours”.9 Glial tumors are envisioned as astrocytic, oligodendroglial, or some combination of these two ("oligoastrocytomas"), as well as special variants or subtypes thereof. These are also graded on a scale of 1 to 4, to reflect expected degrees of aggressiveness.
This is a logical phylogenetic system, if one assumes that these tumors express differentiation toward astrocytes, oligodendrocytes, or both. There is an elephant in the room, however: there is no hard evidence that “oligodendroglial” tumors mimic differentiation toward normal oligodendrocytes. This was simply an historical assumption based upon the observation of some morphologic similarities between “oligodendroglioma” cells and normal oligodendrocytes, coupled with the supposition that normal oligodendrocytes “should” have a neoplastic counterpart. (Note that we are not saying that this assumption is necessarily incorrect—we are simply noting that it remains unproven.) To further complicate the story, there is evidence to suggest that “oligodendrogliomas” arising in the temporal lobe, insula, and diencephalon, are clinicopathologically distinct from oligodendrogliomas arising in the frontal, parietal, and occipital lobes.11
HISTOLOGY OF GLIOMAS
Glial tumors (gliomas) exhibit amazingly varied morphologies. In fact, the most common glial tumor was once known as “glioblastoma multiforme” precisely because its appearance is so variable. Nevertheless, all glial tumors express a glial immunophenotype (a.k.a. antigenic expression), and demonstrate glial differentiation. In the everyday practice of pathology, antigenic expression is one of the most objective means of determining tumor differentiation. Unfortunately, there are no differential antigenic tests to separate “astrocytoma” from “oligodendroglioma”. Rather, there are simply a constellation of soft morphologic features that are suggestive of “oligodendroglial differentiation”, including cellular monomorphism, nuclear roundness, perineuronal satellitosis, secondary structure formation, artifactual cytoplasmic retraction, scanty cytoplasm, calcifications, branching capillaries, and mucin-filled microcysts.
Ironically, "oligodendroglioma" is largely defined by what it lacks—namely glial processes; indeed, the name oligodendroglioma means few processes. Many of the classic morphologic features are consequences of this lack. Also ironically, the most famous microscopic feature (the clear “fried egg” cell) is actually an artifact of slow fixation, which is not found in frozen sections or in rapidly fixed biopsies.
The morphologic features of oligodendroglioma tend to be highly subjective and are fraught with marked interobserver variability. Furthermore, small sample size often exacerbates these problems, since both “astrocytic” and “oligodendroglial” neoplasms of all grades can exhibit regional heterogeneity.
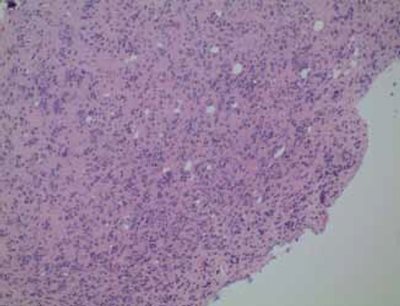
Figure 1. Oligodendroglial neoplasm: Note the marked tendency of tumor cells to cluster around neurons (“perineuronal satellitosis”).
CLINICAL FEATURES OF GLIOMAS
The majority of patients with oligodendrogliomas present with seizures or chronic seizure disorders. The typical patient is a young to middle-aged male with an indolent presentation. Other common presentations include headache, increased intracranial pressure, focal neurologic deficits, and mental status changes.8,9,13
PROGNOSIS AND TREATMENT OF GLIOMAS
Survival statistics vary widely, probably because of lack of universally accepted diagnostic criteria. Median survival is highly dependent upon Grade.4,12,17,18 Low-grade oligodendrogliomas probably have a median survival of greater than 10 years, whereas Grade III oligodendrogliomas (“anaplastic oligodendrogliomas”) have median survival estimates ranging from <1 yr to 3.9 yrs. Grade for grade, “oligodendrogliomas” have a somewhat longer survival than “astrocytomas”, and mixed gliomas (“oligoastrocytomas”) have an intermediate prognosis, although the relative proportions of cell types do not seem to matter.17
Furthermore, approximately 2/3 of oligodendrogliomas have a loss of heterozygosity (LOH) of both chromosome 1p and chromosome 19q. (For a fuller explanation of LOH see http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/T/TumorSuppressorGenes.html Tumors with this dual LOH are exquisitely sensitive to chemotherapy, and treatment with PCV (procarbazine, CCNU aka lomustine, vincristine) may extend survival by 9 or 10 years.2,5,19
Thus, the distinction between “astrocytic” and “oligodendroglial” differentiation is a crucial one and, as noted above, highly subjective. Because we do not want to deny someone the possible benefit of PVC therapy, the trend in neuropathology has been to err on the side of finding oligodendroglial differentiation, probably leading to overdiagnosis.
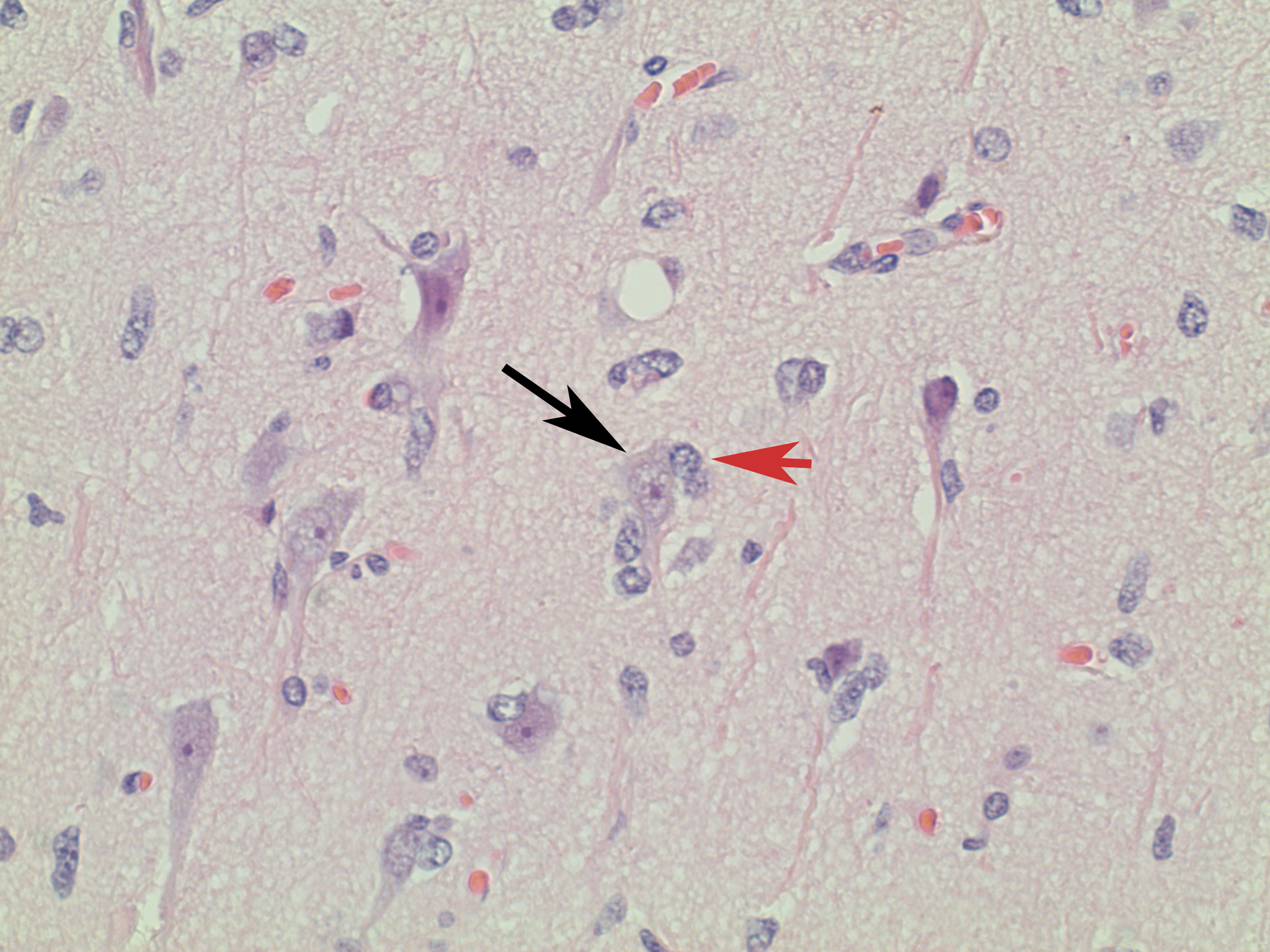
Figure 2. Higher magnification of Figure 1: Note the marked tendency of tumor cells (red arrow) to cluster around neurons (black arrow).
RADIOLOGY OF GLIOMAS
1p/19q codeletion in oligodendrogliomas is associated with an increased frequency of mixed signal intensity on T1- and T2- weighted images.6,9,10 The study by Megyesi et al.10 suggested that 1p/19q codeletions are associated with indistinct tumor borders, paramagnetic susceptibility, and calcification on T1-weighted images; however, the Jenkinson study failed to confirm these associations.
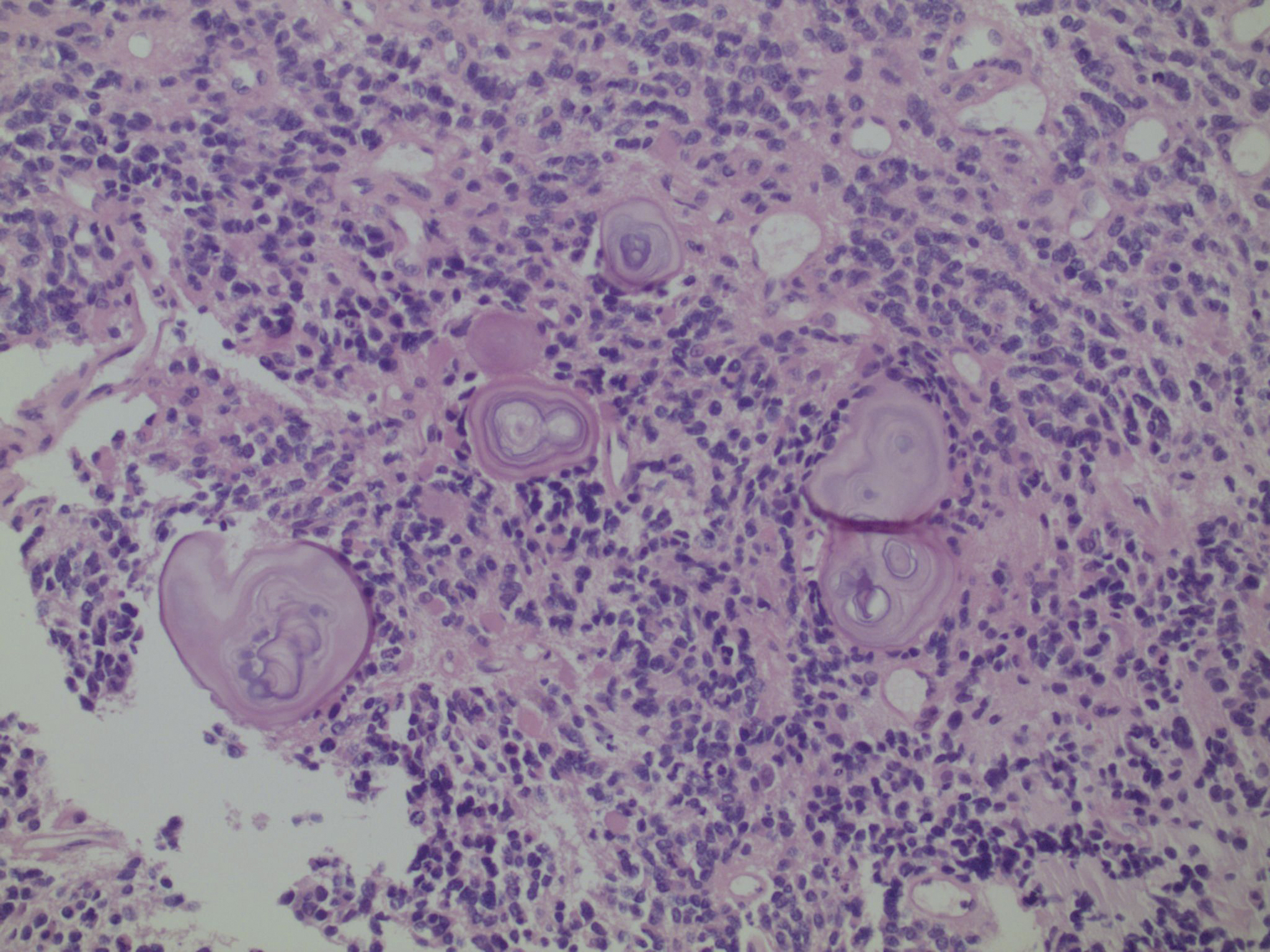
Figure 3. Same neoplasm as Figure 1: Oligodendendroglial neoplasms have a marked tendency to form calcifications. Calcifications are the acellular, whorled, pink-blue laminated structures.
OUR RECOMMENDATIONS REGARDING CYTOGENETIC TESTING
The testing required to diagnose the 1p/19q codeletion costs over $700, and should not be performed indiscriminately, but the extreme difference in response to PVC chemotherapy, and the increased survival, make 1p/19q testing imperative in anyone with a glioma with possible “oligodendroglial” differentiation.
Therefore, we recommend the following:
- Testing should be performed on any glioma with any of the characteristic morphologic findings of oligodendroglial differentiation described earlier under “Histology,” even if only some are present.
- If there is any doubt as to whether testing should be performed, it is better to err on the side of performing the test.
- If the 1p/19q codeletion is present, the patient should be considered a candidate for PCV chemotherapy. It is less clear what to do in the case of oligodendrogliomas lacking this dual deletion as well as in the case of nonoligodendroglial tumors (such as glioblastomas) with this codeletion.
In practice, we tend to diagnose gliomas as astrocytomas (diffuse astrocytoma, anaplastic astrocytoma, or glioblastoma) unless we find definite evidence to support “oligodendroglial” differentiation, in which case the diagnosis becomes either oligodendroglioma or oligoastrocytoma. Lacking this (admittedly highly subjective) evidence, the default diagnosis tends to be astrocytoma.
Instead, it may be more productive when faced with a glial neoplasm to start from the assumption that analysis for the 1p/19q codeletion is merited and then look for morphologic evidence to disprove this assumption.
This approach represents a paradigm shift: rather than looking for evidence of “oligodendroglial” differentiation, we are looking for evidence that genetic testing will NOT benefit the patient. Lacking such definitive evidence, 1p/19q testing becomes the default option. We believe that this is in the patient’s best interest, because if there is any uncertainty, the patient will receive the benefit of the doubt.
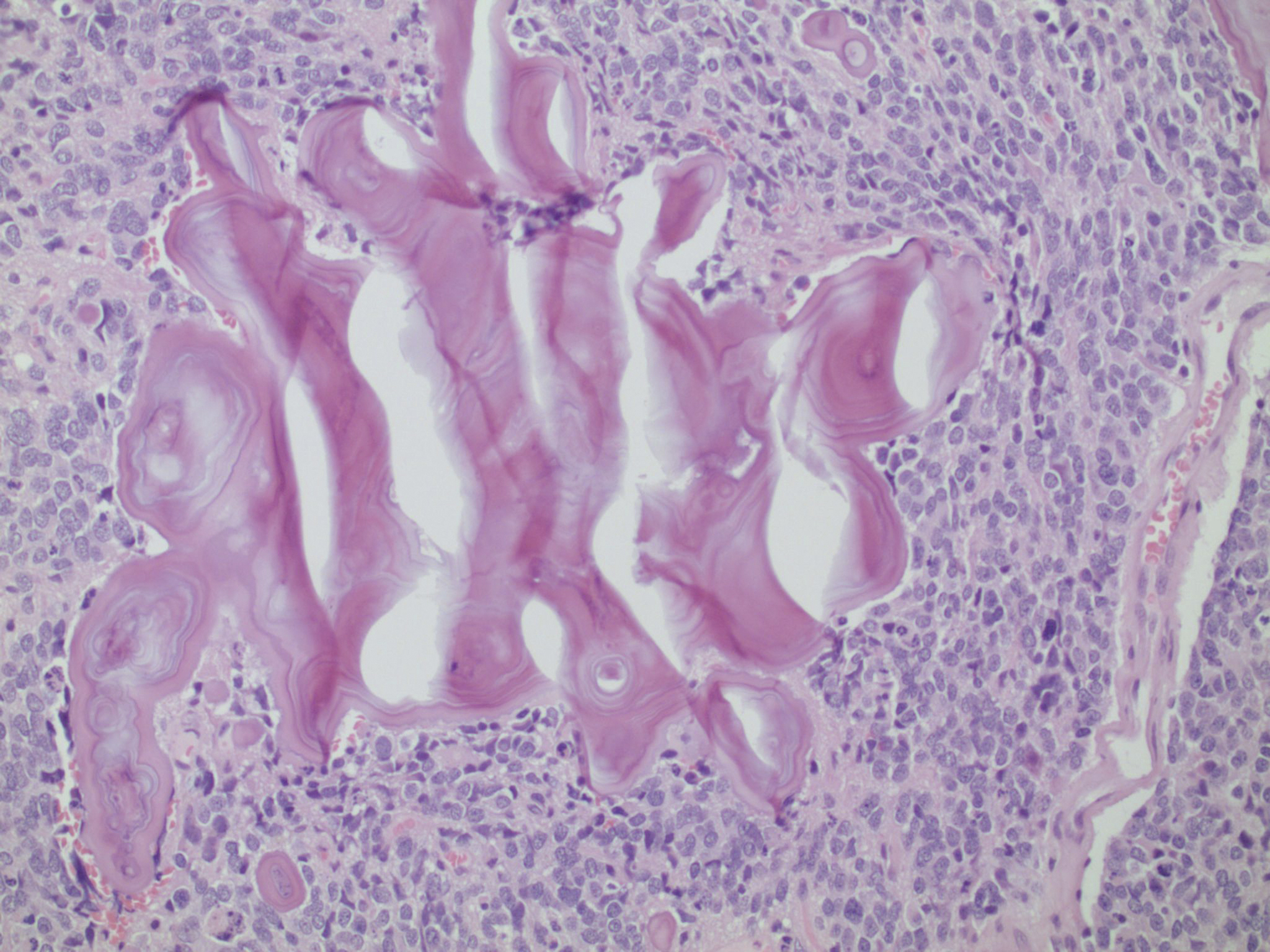
Figure 4. Same neoplasm as Figures 1: Calcifications can form complex structures with unusual geometric shapes.
SUMMARY
Our department has been looking for some guidance as to when to test for the 1p/19q dual deletions. On the one hand, we do not want to order testing indiscriminately, especially since each test costs over $700. On the other hand, given the strong predictive and prognostic values of the test in the appropriate patients, we feel that if there be any doubt, we would rather err on the side of doing the test.
We agree with Perry’s conclusions in his masterpiece review of oligodendroglial neoplasms (a must-read for all oligodendrogliomologists)14 that both clinicopathologic and genetic findings likely provide far more accurate stratification than either one alone.15 Their prognostic value in pure oligodendrogliomas is clear, though our understanding of the relative importance of histologic vs. genetic data in determining therapy is still evolving. It is, nevertheless, clear that 1p/19q codeletion is relatively specific for oligodendrogliomas with long survival.
The literature seems fairly clear that as long as oligodendroglial differentiation is present (either pure oligodendroglioma or mixed glioma with an oligodendroglial component) 1p/19q testing should be performed, regardless of grade. However, to our dismay, it also became clear upon reviewing the literature that neither light microscopy nor immunophenotyping provides a reliable morphology criterion for separating oligodendroglial from non-oligodendroglial glial differentiation, and we see nothing on the horizon to suggest that this quandary will be resolved any time soon.
BIBLIOGRAPHY
1. Bishop M, de la Monte SM. Dual lineage of astrocytomas. Am J Pathol 1989; 135: 517-529.
2. Cairncross JG, Ueki K, Zlatescu MC, Lisle DK, Finkelstein DM, Hammond RR, Silver JS, Stark PC, Macdonald DR, Ino Y, Ramsay DA, Louis DN. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst 1998; 90: 1473-1479.
3. De la Monte SM. Uniform lineage of oligodendrogliomas. Am J Pathol 1989; 135: 529-540.
4. Dehghani F, Schachenmayr W, Laun A, Korf H-W. Prognostic implication of histopathological, immunochemical and clinical features of oligodendrogliomas: a study of 89 cases. Acta Neuropathol 1998; 95: 493-504.
5. Fuller CE, Schmidt RE, Roth KA, Burger PC, Scheithauer BE, Banerjee R, Trinkaus K, Lytle R, Perry A. Clinical utility of fluorescence in situ hybridization (FISH) in morphologically ambiguous gliomas with hybrid oligodendroglial/astrocytic features. J Neuropathol Exp Neurol 2003; 62: 1118-1128.
6. Jenkinson MD, du Plessis DG, Smith TS, Joyce KA, Warnke PC, Walker C. Histological growth patterns and genotype in oligodendroglial tumours: correlation with MRI features. Brain 2006; 129: 1884-1891.
7. Larsen WJ. Human Embryology 2nd ed. 1997; New York: Churchill Livingstone. p.90.
8. Lebrun C, Fontaine D, Ramaioli A. Vandenbos F, Chanalet S, Lonjon M, Michiels JF, Bourg V, Paquis P, Chatel M, Frenay M and The Nice Brain Tumor Study Group. Long-term outcome of oligodendrogliomas. Neurology 2004; 62: 1783-1787.
9. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. WHO Classification of tumours of the central nervous system, 4th ed.. 2007. International Agency for Research on Cancer.
10. Megyesi JF, Kachur E, Lee DH, Ziatescu MC, Betensky RA, Forsyth PA, Okada Y, Sasaki H, Mizoguchi M, Louis DN, Cairncross JG. Imaging correlates of molecular signatures in oligodendrogliomas. Clin Cancer Res 2004; 10: 4303-4306.
11. Mueller W, Hartmann C, Hoffmann A, Lanksch W, Kiwit J, Tonn J, Veelken J, Schramm J, Weller M, Wiestler OD, Louis DN, von Deimling A. Genetic signature of oligoastrocytomas correlates with tumor location and denotes distinct molecular subsets. Am J Pathol 2002; 161: 313-319.
12. Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol 2005; 64: 479-489.
13. Olson JD, Riedel E, DeAngelis LM. Long-term outcome of low-grade oligodendroglioma and mixed glioma. Neurology 2000; 54: 1442-1448
14. Perry A. Oligodendroglial neoplasms: Current concepts, misconceptions, and folklore. Adv Anat Pathol 2001; 8: 183-199.
15. Perry A, Fuller CE, Banerjee R, Brat DJ, Scheithauer BW. Ancillary FISH analysis for 1p and 19q status: preliminary observations in 287 gliomas and oligodendroglioma mimics. Frontiers in Bioscience 2003; 8: a1-a9.
16. Schiffer D, Dutto A, Cavalla P, Bosone I, Chio A, Villani R, Bellotti C. Prognostic factors in oligodendroglioma. Can J Neurol Sci. 1997 24: 313-319.
17. Shaw EG, Scheithauer BW, O’Fallon JR, Davis DH. Mixed oligoastrocytomas: a survival and prognostic factor analysis. Neurosurgery 1994; 34: 577-582.
18. Shaw EG, Scheithauer BW, O’Fallon JR, Tazelaar HD, Davis DH. Oligodendrogliomas: the Mayo Clinic experience. J Neurosurg 1992; 76: 428-434.
19. Smith JS, Perry A, Borell TJ, Lee HK, O’Fallon J, Hosek SM, Kimmel D, Yates A, Burger PC, Scheithauer BW, Jenkins RB. Alterations of chromosome arms 1p and 19q as predictors of survival in oligodendrogliomas, astrocytomas, and mixed oligoastrocytomas. J Clin Oncol 2000; 18: 636-645.
|
|