Abstract
Methicillin-Resistant Staphylococcus aureus (MRSA) has been a recognized pathogen for almost half a century. Until recently, MRSA was confined predominately to hospitals, nursing homes, and other healthcare facilities that care for debilitated and chronically ill patients (Healthcare-Associated, or HA-MRSA). In the last decade, however, MRSA has caused rapidly increasing numbers of invasive infections in young and otherwise healthy persons across the globe. This new pathogen is Community-Acquired (CA-MRSA) .
This latest epidemic is not due to spillover of hospital strains into the community, but rather represents a new and unique step in microbial evolution. This paper discusses the unique genetic makeup, virulence factors, epidemiology, and clinical manifestations of this new pathogen. Contemporary approaches to the diagnosis and current treatment of CA-MRSA are also discussed.
Introduction
In 1961, the penicillinase-resistant antibiotic methicillin was introduced to combat strains of penicillin-resistant Staphylococcus aureus (SA)that had become prevalent since the mid-1940’s. Within one year, methicillin-resistant strains of SA (MRSA) had evolved in the United Kingdom, the United States, Europe, and Australia.1 This strain gradually gained ascendancy during the 1970’s, and is in fact still the major genotype for the traditional HA-MRSA that remains endemic in health care settings around the world. In the late 1990’s, however, atypical MRSA cases began to appear increasingly in young and healthy patients in large metropolitan centers, and has since become an epidemic across the USA and around the world. This increased caseload is now known to be due to a new microbe: community-acquired MRSA (CA-MRSA).
Genetics of MRSA
To understand the genesis of this new microbe, a brief review of the genetics of methicillin resistance in SA is necessary. The property of methicillin resistance in SA is contained in mobile islands of self-regulating genetic material in the cytoplasm. These are called Staphylococcal Chromosomal Cassettes, or SCCmec genes, of which there are multiple subtypes (Table 1). The initial strains of HA-MRSA that appeared in the 1960s, and predominated through the next few decades, were clonally derived from SCC mecA. Recall that penicillin’s antimicrobial activity stems from its interference with cell-wall synthesis. Without going into laborious detail, SCC mecA encodes for a binding protein that has abnormally low affinity for penicillin (PBP2a), and thus allows cell wall synthesis in the presence of penicillins or cephalosporins. SCCmec gene subtypes II and III also contain linked genes that mediate resistance to erythromycin, clindamycin, neomycin, and quinolones, and in combination produce a predictable phenotype for the then-circulating MRSA strains. For the genomics maven, a more detailed discussion can be found in the review by Crawford.2
SCC mec type IV
The linchpin event responsible for the new epidemic of CA-MRSA was the evolution of a novel mec gene, SCCmec type IV. Unlike its predecessor mec genes, SCCmec type IV is not linked to the genes that confer resistance to non-beta-lactam antibiotics. Thus CA-MRSA is characteristically sensitive to erythromycins, clindamycin, or quinolones; sensitivity to tetracyclines and trimethoprim-sulfa also tends to be preserved. However, the SCC mec type IV gene is associated with multiple potent factors that mediate virulence, and are responsible for the clinically aggressive nature of CA-MRSA infections.
Panton- Valentine Leukocidin
In a case of rapidly fatal CA-MRSA sepsis in a 16 month old, detailed genomic analysis yielded 19 virulence genes not characteristically found in HA-MRSA strains.3 While various enterotoxins, leukotoxins, and superantigens have been described in CA-MRSA isolates, the most potent, and the most characteristic is the Panton-Valentine Leukocidin (PVL). This unique bacteriophage-mediated toxin, which is highly associated (although not linked) with the SCCmec IV gene, has numerous characteristics that account for the substantial morbidity and mortality of CA-MRSA: it is toxic to neutrophils, and causes tissue necrosis, invasive cutaneous disease, and necrotizing pneumonia.4 PVL is only rarely found in HA-MRSA strains, and it is characteristically absent in patients colonized with CA-MRSA who do not progress to invasive infection.
Epidemiology of CA-MRSA
As noted earlier, during the last decade MRSA infections have appeared in populations outside of those traditionally associated with HA-MRSA. Initial reports involved outbreaks in children, particularly those in day care,5 and often with a history of exposure to other ill siblings or schoolmates. Outbreaks in members of high school, college, and professional sports teams soon followed.6 Contact sports predominated, with football and wrestling at the forefront. Turf burns and communal whirlpools were identified as risk factors. Sharing of equipment in non-contact sports is also risky, as an outbreak occurred in a college fencing team. Overcrowding of young healthy persons also carried some risk, as reported in outbreaks among prison inmates7 and military recruits.8
In addition to the above distinctive epidemiologic features, CA-MRSA is further differentiated from HA-MRSA by its clinical presentation and antibiotic susceptibility. Classification systems based on pulse-field gel electrophoresis have been devised, and specific strain lineage types have been identified. The predominant strain in America is designated USA 300, which carries the mecB SSC type IV genotype, and is positive for PVL. Since laboratory identification schemes with such detail are beyond the reach of most hospitals, the CDC has devised a useful clinical case definition (Table 2).
Initial reports of CA-MRSA initially appeared in the late 1990’s, but since 2002 a more acute rise in CA-MRSA cases has occurred in the U.S.9 Again, a higher incidence in children less than two years of age has been noted, and the majority of cases involve skin site infections, including necrotic pustules, abscesses, and cellulitis. Hospitalization has been required in over 20% of patients, with increased morbidity and mortality compared to HA-MRSA or to methicillin-sensitive Staphylococcus aureus (MSSA).
CA-MRSA in Lancaster County
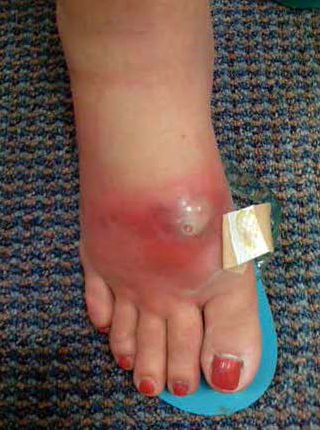
The diffusion of resistant microbes to Lancaster County from urban centers has characteristically been delayed several years. While CA-MRSA has been described in urban centers since the late 1990’s, the epidemic curve in Lancaster County did not begin to rise rapidly until 2005 (Figure 1). Nonetheless, the disease burden of CA-MRSA in the past year has risen to over 350 cases, even while the background endemic rate for HA-MRSA has increased slowly to an average of only 45 cases annually.
Colonization vs. Infection
Conventional wisdom about human bacterial skin flora has been redefined by recent studies that utilize sensitive genomic techniques.10 The average human skin surface contains 50-500 thousand bacteria per square inch, consisting of an average of 180 different species which vary according to age, sex, body part, season, and geography. Gram-positive bacteria predominate because Gram-negative microbes are inhibited by surface fatty acids. (A notable exception is Actinobacter, which caused endemic infections in Gulf War participants.)
About 30% of the general human population is colonized with SA at any given time,11 usually in the anterior nares. The pattern of colonization tends to follow the ‘20-60-20 rule.’ Twenty percent of humans are colonized almost continuously, another 20% are almost never colonized, and the 60% majority demonstrates transient colonization of the nasopharynx for weeks to months, alternating with periods of non-colonization. The predominant method of spread is human skin-to-skin contact, but transmission via inanimate objects, or fomites, also occurs. The likelihood and persistence of colonization with SA is increased in the setting of chronic medical illness, such as obesity, diabetes, or chronic dermatitis.
Recent epidemiologic studies have demonstrated that 46 of every 1,000 hospitalized patients are colonized or infected with MRSA.12 The vast majority of HA-MRSA is imported, mainly from extended care facilities or from readmission of patients previously colonized or infected with MRSA. More interesting still, 6% of health care workers (HCW) on acute care units, and up to 36% on long-term care units, may be colonized with MRSA. Family members of HCW may also be colonized with the same strain of MRSA as the related HCW.13
Community epidemiologic studies estimate that 1-9% of the United States population is now colonized with CA-MRSA,9 depending on geography and age. CA-MRSA colonization rates also seem to be increasing with time. In a longitudinal study in Tennessee, childhood colonization rates increased from 0.8% in 2001 to 9.2% in 2004.11
In persons colonized with CA-MRSA, the risk of invasive infection is greater than with either HA-MRSA or MSSA. In a study of military recruits, 38% of those colonized with CA-MRSA developed infection within 8 weeks, compared to 3% for those with MSSA colonization, a relative risk of 10.7.8 In addition, the presence of a PVL-positive strain is an independent predictor of progression to infection.
CLINICAL FEATURES
Skin and Soft Tissue (SST) syndromes
Approximately 80% of CA-MRSA infections present as skin and soft tissue infections.2 The most characteristic lesion is the ‘spider bite’ (Figure 2.), a raised tender erythemetous nodule, often with a necrotic center, that is frequently confused with actual arachnid bites, especially that of the brown recluse spider. (Since this spider is not found in the Northeast, the attribution is ill founded.) It is very painful, often being described as seeming like ‘someone holding a match to my skin.’ Commonly associated characteristics include (Figure 3), abscess formation, lymphangitis, lymphadenitis, fever, and constitutional symptoms. Lesions may be multiple or migratory, and can sometimes resolve spontaneously. Isolated strains are almost always the USA-300 genotype, and are positive for the PVL toxin.
Invasive disease syndromes
Deeper invasion of CA-MRSA can lead to fasciitis, myositis, and osteomyelitis. Bacteremia, acute endocarditis, and metastatic foci of infection may occur. Toxic shock syndrome, septic shock, and death have also been described. The time course from a surface lesion to invasive disease may – at worst - be only a matter of hours, and overall tends to be more rapid than with MSSA infections.2 CA-MRSA has been described as a cause of fatal community-acquired pneumonia, especially post-influenza.14
Diagnostic Methods
Identification of CA-MRSA is identical to that for HA-MRSA. Cultivation of clinical specimens utilizes a screening agar containing NaCl and 4 micrograms/ml of oxacillin. Growth and confirmation take 48-72 hours by standard methods, a delay in identification that is problematic from the standpoints of appropriate diagnosis, institution of appropriate therapy, and quarantine. New Polymerase Chain Reaction (PCR) methodology promises to provide rapid identification of patients colonized or infected by MRSA.15 Several diagnostic kits are now commercially available, with results available in hours rather than days. While these kits are FDA-approved only for nasal swab specimens at the present time, their use will be a leap forward.
Treatment of CA-MRSA
Topical Treatments
Standard soaps, even those labeled ‘anti-bacterial,’ have little effect on colonization with MRSA. Topical chlorhexidine soaps at 2-4% concentration, however, are bactericidal for CA-MRSA. Although formal studies are lacking, use of these soaps thrice weekly can provide both treatment of minor skin lesions, as well as decolonization of the skin surface. In a few patients with very superficial lesions, this regimen may suffice for treatment.
Systemic Therapy
Fortunately, the treatment options for CA-MRSA are significantly broader than for HA-MRSA, due to the absence of linkage between genes for antibiotic resistance and SCCmec IV. Antibiotics that are usually effective include clindamycin, the erythromycin derivatives, some quinolones, doxycycline, and trimethoprim-sulfamethoxazole. In appropriate doses, oral administration provides adequate tissue levels to treat mild to moderate cellulitis and pustular lesions in afebrile, non-toxic patients. Randomized controlled trials are lacking, unfortunately, so that no specific agent or regimen can be favored. The additional use of topical chlorhexidine and mupirocin should be considered.16
For patients with suspected sepsis or bacteremia, and for those with more advanced cutaneous disease, such as deep abscess or myofasciitis, parenteral antibiotic therapy is indicated, together with appropriate surgical incision and drainage of infected tissues. While treatment of invasive or systemic infection usually involves the use of vancomycin, many studies have suggested that, at least at standard doses, it may be suboptimal for invasive MRSA infections.2
More recently, additional compounds have been introduced which show specific promise for the treatment of severe life- or limb-threatening MRSA infections. These include linezolid, daptomycin, and tigecycline, as well as quinupristin-dalfopristin, a less favored, but still effective older drug. At Lancaster General Hospital, these antibiotics are appropriately part of a restricted formulary to minimize overuse and consequent evolution of resistance.
No studies exist to define the best agent or exact duration of treatment for CA-MRSA infections, but clinical experience favors a more prolonged course than for similar MSSA infections. In addition, the possibility of re-exposure and re-infection through re-inoculation of patients from colonized family members remains problematic.
Adjunctive Therapy
Toxin-mediated infections such as group A streptococcal and staphylococcal toxic shock syndromes respond to adjunctive therapy with intravenous immunoglobulin (IVIG), probably because binding of circulating superantigens ameliorates the ‘cytokine storm’ that is key to the pathogenesis of those syndromes. As PVL-positive CA-MRSA infections, such as necrotizing pneumonia, are thought to develop via the same mechanism, the use of IVIG as been suggested. In vitro data, in fact, demonstrate that PVL can be neutralized by some preparations of IVIG, and further clinical trials are being developed.17
Prevention
The model of spread for CA-MRSA is the same as for all strains of SA: nasal colonization with skin-to-skin transmission. Interventions aimed at these two foci have been proposed as preventive measures.
Mupirocin ointment, applied to the nares bid for 5 days, is an effective regimen for nasal decolonization with SA,16 but its effectiveness for CA-MRSA is not clearly established, eradication may be short-lived, and development of resistance may ultimately limit its use.2 Nonetheless, the use of mupirocin both for patients and household contacts of infected patients is probably justified at this time.
Attempts to limit spread from person-to-person center primarily on hand hygiene and wound care. Alcohol-based hand sanitizers are effective, and are routinely utilized pre- and post patient care in hospitals and clinics. In the setting of athletics, the CDC has published guidelines to limit the sharing of towels, equipment, and whirlpools, and to maximize hygiene through the availability of bactericidal soaps. Wound care guidelines designed to ensure proper disposal of contaminated items and barrier care have also been released. Educational materials about skin lesion screening are also available. The CDC has issued an excellent summary of community management of CA-MRSA.18
In the hospital setting, prompt contact isolation of all patients suspected of MRSA infection or colonization is crucial to containment. Strict adherence to proper procedures by all hospital personnel and visitors is difficult but of the utmost importance, along with continuing education of patients and their families.
Future Directions
The CA-MRSA epidemic is upon us, and is here to stay.12 Its sudden, striking explosion on the health care scene has caught us off guard, and much research is still needed to define the proper approach to management of both infected and colonized patients. Antibiotic utilization must be rigorously scrutinized to minimize selection pressures on local flora. The proper use of chlorhexidine and mupirocin for decolonization must be researched and defined. Comparative trials to define optimal antibiotic agents and treatment regimens need to be undertaken as well.
The advent of rapid molecular (Polymerase Chain Reaction) diagnostic methods is exciting.15 A program for the rapid detection of MRSA through PCR techniques is currently being formulated at LGH and other institutions. Its implementation, along with proper adherence to isolation protocols, will have many potential benefits, including minimization of nosocomial spread by rapid screening of high-risk patients, and screening of pre-operative patients in hopes of curtailing post-operative infection. Future uses (requiring a change in FDA indications) could include rapid identification of MRSA in clinical specimens (wounds, blood, body fluids) to optimize protocols for both isolation and treatment.
Addressing the epidemic at the community level, however, may prove to be a complex dilemma that can only be resolved by action at various points, and the public’s fear must be allayed with information. Certainly, broad-based education is needed, and the current focus on student athletes must be expanded to include educational programs for all students at all levels. Insurance carriers need to recognize that their endorsement and coverage of education, screening, and treatment will benefit the public health. Finally, government must make education, diagnosis, and treatment of CA-MRSA infections a priority.
REFERENCES
- Barber, M. Methicillin-resistant staphylococci. J Clin. Pathol. 1961; 14:385.
- Crawford, SE Et al. Community-Acquired Methicillin-Resistant Staphylocccus aureus. Emerging Infections. 2007. 7(9):153.
- Four Pediatric Deaths from community-acquired methicillin-resistant Staphylococcus aureus : Minnesota and North Dakota, 1997-1999. MMWR. 1999; 48:707.
- Lina, G. Et al. Involvement of the Panton-Valentine Leukocidin-producing Staphylococcus aureus in primary skin infection and pneumonia. Clin. Infect. Dis. 1999; 29:1128.
- Herold, BC Et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA 1998;279:593.
- Methicillin-resistant Staphylococcus aureus infections among competitive sports participants – Colorado, Indiana, Pennsylvania, and Los Angeles County, 2000-2003. MMWR. 2003;52:793.
- Methicillin-resistant Staphylococcus aureus infections in correctional facilities –Georgia, California, and Texas 2001-2003. MMWR 2003;52:992.
- Ellis, MW., et al. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin. Infect. Dis. 2004; 39:971.
- Fridkin. Et al. Methicillin-Resistant Staphylococcus aureus Disease in Three Communities. NEJM 2005;352(14)
- Gao, Z et al. Molecular analysis of human forearm superficial bacterial biota. Proc. Natl. Acad. Sci. USA 2007;104(8):2927
- Creech, CB. Et al. Increasing rates of nasal carriage of methicillin-resistant Staphylococcus aureus in healthy children. Pediatric Infect. Dis. J. 2005;24:617.
- National Prevalence Study of Methicillin-Resistant Staphylococcus aureus (MRSA) in U.S. Healthcare Facilities. 2007, Association for Professionals in Infection Control and Epidemiology.
- Eveillard, M. Et al. Carriage of methicillin-resistant Staphylococcus aureus among hospital employees: Prevalence, duration, and transmission to households. Infect. Control Hosp. Epidemiol. 2004; 25(2):114.
- Hageman, JC. Et al. Severe community-acquired pneumonia due to methicillin-resistant Staphylococcus aureus, 2003-4 influenza season. Emerging Infect. Dis. 2006;12:894
- Cunningham, R. et al. Effect on MRSA transmission of rapid PCR testing of patients admitted to critical care. J. Hosp. Infection. 2006;65(1):24.
- Doebbeling BN, el al. Elimination of Staphylococcus aureus nasal carriage in health care workers: analysis of six clinical trials with calcium mupirocin ointment. Clin. Infect. Dis. 1993; 17:466.
- Gauduchon, VG, et al. Neutralization of Staphylococcus aureus Panton-Valentine leukocidin by intravenous immune globulin in vitro. J. Infect. Dis. 2004; 189:346
- Strategies for Clinical Management of MRSA in the Community: Summary of an Experts’ Meeting Convened by the CDC. 2006 (www.cdc.gov/ncidod/dhap/pdf/ar/CAMRSA ExpMtgStrategies.pdf.)
Joseph M. Kontra, M.D.
Infection Specialist of Lancaster
2106 Harrisburg Pike, Suite 301
Lancaster, PA 17601
717-544-3517
jmkontra@LGHealth.org
Special Reviewer’s Comment by Andrew S. Coco, M.D.
I applaud Dr. Joseph Kontra’s efforts to raise our awareness of Community-Acquired MRSA. Over the past year, the LGH Research Institute has been studying how physicians modify inpatient antibiotic regimens in accordance with blood culture susceptibility results. Because antimicrobial resistance is continuing to increase in health care settings, the Centers for Disease Control and Prevention in 2003 developed a national 12-step program entitled, “Campaign to Prevent Antimicrobial Resistance in Healthcare Settings.” Step 3 of this program—Target the Pathogen—calls for performing cultures and targeting therapy to known pathogens in accordance with antimicrobial susceptibility tests. The objective of our study is to determine the extent to which LGH physicians are choosing antibiotics based on the results of sensitivity tests of positive blood cultures.
The preliminary results were presented by Scott Delong MD, Shelby Margut MD, and Megan Thomas MD at the Family Medicine Research Day on June 12th. There are clearly opportunities for improvement. A review of 80 patient records from 2005 revealed that even after susceptibility results became available, 67% of the antibiotic choices were categorized as excessive, meaning that narrower spectrum choices were available but were not utilized. Although that study is concerned with hospitalized patients, similar tendencies may well be prevalent in outpatient settings where CA-MRSA is important.
I urge all health care providers to carefully consider their antibiotic choices in both the hospital and outpatient settings, and when feasible, to utilize the narrow spectrum agents often recommended in clinical guidelines published by specialty and public health organizations. In this way we can minimize selection pressure on local flora and not only decrease the number of CA-MRSA infections but also help to prevent the next resistant pathogen from emerging in our community.
Table 1. Characteristics of MRSA Genetic Subtypes
|
SCCmec
|
mec Complex
|
linked resistance genes
|
PVL
|
|
I
|
mecB
|
++
|
-
|
|
II
|
mecA
|
+++
|
-
|
|
III
|
mecA
|
+++
|
-
|
|
IV
|
mecB
|
-
|
+++
|
Table 2. Centers for Disease Control CA-MRSA Case Definition
A. Positive MRSA culture obtained as outpatient or within 48 hours of admission.
B. Patient has no history of hospitalization, dialysis, surgery, IV therapy, or residence in a long-term care facility during the year before infection.
C. Patient has no prior history of injection drug use, or prior MRSA colonization or infection
Figure 1. MA-MRSA/CA-MRSA
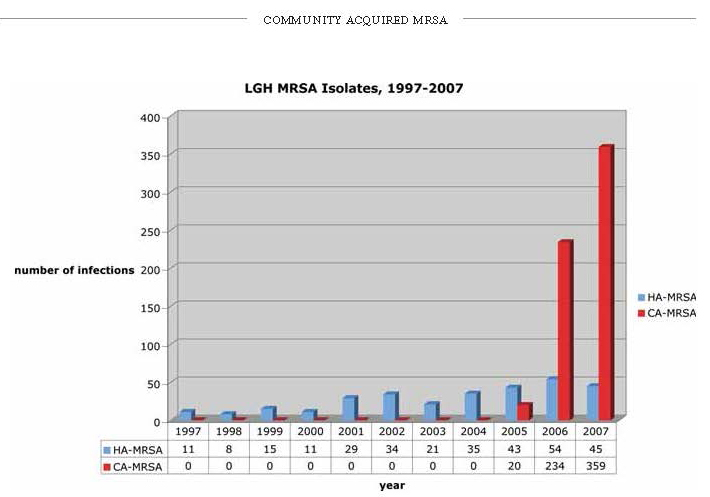
Figure 2. typical “spider bite” lesion of CA-MRSA
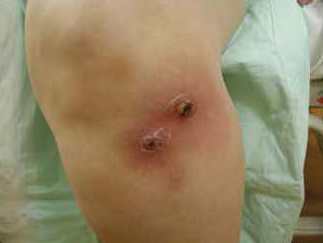
Figure 3. Spontaneous abscess and cellulitis caused by Community-Acquired MRSA.