Carotid endarterectomy (CEA) has been shown to be significantly better than medical therapy in the Asymptomatic Carotid Atherosclerosis Study, and the North America Symptomatic Carotid Endarterectomy Trial. Notably, patients in these trials were considered low risk, and high risk patients were excluded.*
*Editor’s note: Early trials of carotid endarterectomy had such a great impact because they demonstrated benefit even in patients at low risk, in whom it is more difficult to demonstrate a therapeutic benefit. A cohort at higher risk than the general population magnifies the significance of any therapeutic benefit.
Against the background of proven benefit for surgical revascularization, and the success of interventional techniques elsewhere in the vascular system, a non-invasive interventional approach to carotid stenosis was developed and evaluated in extensive research and clinical trials. Ultimately, the FDA approved carotid artery stenting (CAS) with distal protection as another option for high risk patients who could not undergo surgical endarterectomy safely. Minimally invasive CAS has now emerged as a promising modality for stroke prevention due to its low peri-procedural complication rate and midterm durability. It also avoids any threat of cranial nerve injury.
Carotid stenting is performed like a standard catheterization procedure using local anesthesia. Following needle puncture of a groin artery, a catheter and wire are navigated under x-ray guidance to the carotid artery where a small filter-like device is delivered downstream from the vessel’s obstruction to “catch” debris that could potentially become dislodged during the procedure. The distal protection device helps to prevent debris from entering the brain and causing a stroke. (The internal carotid artery beyond the stenosis must be large enough to accommodate the filter – approximately 3.5-5.5 mm.) Next, an expandable balloon is momentarily inflated to provide a pathway through the narrowing, and a self-expanding stent is deployed across the narrowed artery to help restore normal blood flow. The protection device and other equipment are retrieved and discarded and the stent remains in place. The patient typically stays overnight for observation but is discharged the next morning, and may return to normal activities the following day.
A clinical trial called the SAPPHIRE trial (Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy) demonstrated that in selected patients at high risk for surgery, carotid stenting is a safe and equally effective alternative to carotid endarterectomy (CEA). Patients deemed at high risk included those with any of the following: age older than 80; severe heart or lung disease; complete blockage of one carotid artery; tandem stenoses (one in the neck and the other intracranial); laryngeal nerve palsy; prior neck surgery; or radiation therapy to the neck. In such patients, the SAPPHIRE study data favored carotid artery stenting, with a significant reduction in stroke, death, and heart attack compared with patients who underwent surgery.* The need for additional revascularization procedures and the length of the hospital stay was also less in the stent group compared with the surgery group. The overall rate of complications in the SAPPHIRE trial was 4.8% for stenting and 9.6% for surgery.
*Editor’s note: As discussed in the article by Mangeshkumar in this issue, 55% of patients in the SAPPHIRE trial who were poor candidates for surgery were excluded from randomization and were entered into a prospective registry. In more than 20% of patients in each group, the indication for surgery was restenosis following prior CEA, which made them better suited for endovascular treatment.
The BEACH trial (Boston Scientific EPI-A Carotid Stenting Trial for High Risk Surgical Patients) has now provided two year data which show that carotid stenting is a durable treatment which actually decreases a patient’s risk of stroke and death compared with surgery.* Controlled studies are now underway to evaluate CAS versus CEA, as well as CAS in other patient populations.
*Editor’s note: The BEACH trial was not a randomized study with a control group that underwent surgery, but rather a prospective registry in which results of CAS were compared with objective performance criteria derived from historical controls who underwent CAE.
Results of the SAPPHIRE and BEACH trials, which were carried out in high risk patients, cannot be generalized to patients at low risk. Additional trials are being conducted to assess the appropriateness of stenting in lower risk patients, but carotid artery stenting can clearly be offered to patients who are at great risk for having a stroke but are also at high risk for surgery because of the risk factors enumerated earlier.
Carotid artery stenting is currently being performed at Lancaster General Hospital in the Angiography and Interventional Radiology division with increasing frequency due to the advancing age of the community, and the wide spectrum and complexity of diseases treated locally.
John Briguglio, M.D.
Staff Radiologist, Lancaster General Hospital
Lancaster Radiology Associates, Ltd.
P.O. Box 3555
Lancaster, PA 17604
717-544-4900
JOBrigug@LGHealth.org
Leigh S. Shuman, M.D.
Staff Radiologist, Lancaster General Hospital
Lancaster Radiology Associates, Ltd.
P.O. Box 3555
Lancaster, PA 17604
717-544-4900
LSShuman@LGHealth.org
Imaging Insights
Legends for figures:
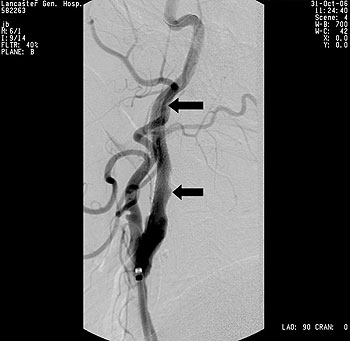
Figure 1 – High grade stenosis (arrow) of the right internal carotid artery in a patient with a high lesion and multiple comorbidities.
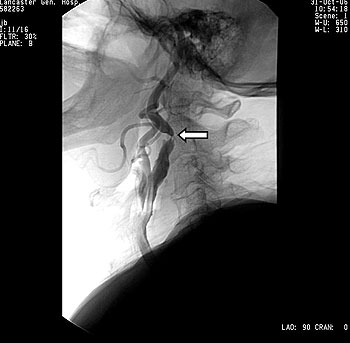
Figure 2 – A balloon (arrow) expands the stent in the right internal carotid artery.

Figure 3 – Final result with stent in place. The arrows mark the proximal and distal ends of the stent.