Summer 2020 - Vol. 15, No. 2

 Small Madara
Small Madara
Spotlight on Clinical Research
COVID-19 Research Update
Roy S. Small, M.D.
Medical Director of Clinical Research
Heather Madara
Clinical Research Coordinator
Penn Medicine Lancaster General Health Research Institute
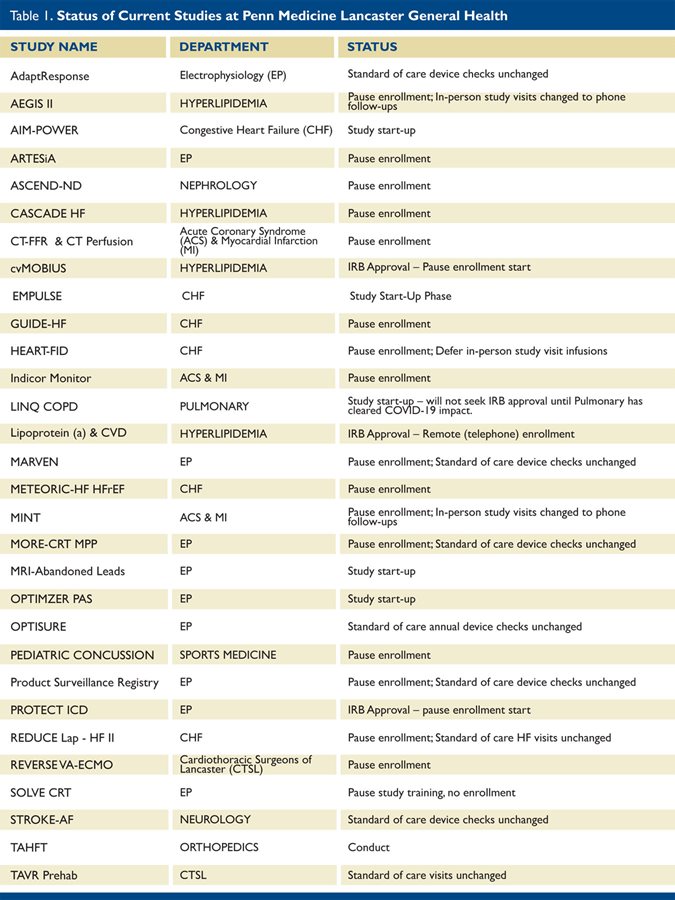
The management committee of the Research Institute met in March to determine how the team would approach research studies during the COVID-19 pandemic. In order to maximize patient and staff safety and continue as many operations as possible, the Research Institute implemented the following recommendations:
1. Research studies which require hospital resources will temporarily cease enrollment unless the procedure is part of standard medical care. For example, MORE-CRT MPP requires implanting an ICD.
2. Studies requiring inpatient visits for enrollment (for example, the MINT trial, which involves transfusions in ACS patients) will be temporarily placed on hold.
3. Follow-up visits for research patients will be done remotely (by telephone) if possible, instead of in the office.
4. Research patients requiring face-to-face visits will have follow-up visits scheduled per protocol. Similarly, patients receiving research drugs will continue to receive these drugs per present protocols. Some studies, such as ARTESiA, were granted approval to ship study drugs directly to the patients during this time.
5. Procedures that can be delayed yet remain in the prescribed protocol “window” (for example, HEART-FID in which patients receive iron infusions) will be rescheduled.
6. The Research Institute notified the IRB, all study sponsors, and Principal Investigators (study PI’s) of these changes in writing. The study PI’s signed off on the interim plans.
The following table identifies the status of all current studies and their departments.