 Click to Print Adobe PDF
Click to Print Adobe PDF
Fall 2011 - Vol.6, No.3
Hypothermia for
Neuroprotection in the Newborn
Janet E. Larson, M.D.
Dorothy McElwee, MSN, NNP-BC
Nemours
A.I. duPont Hospital for Children
|
  |
Introduction
Perinatal hypoxic-ischemic encephalopathy is a major source of morbidity and mortality throughout the world.1-4 Until recently, there was little to offer these infants except for supportive care, but there are now increasing data that support the use of moderate prolonged hypothermia to reduce cerebral injury and improve neurological outcome in these infants.5 There are two therapeutic modalities that have been trialed and are now in use; selective cooling (achieved by decreasing body temperature to 34.5° C with the use of a cap applied to the head) and whole body cooling, which lowers the temperature of the body to 33.5° by use of a cooling blanket. Both methods show evidence of therapeutic benefit in improving survival and reducing the rate of disability at 18 months of age in newborns.5
What is Encephalopathy?
Encephalopathy is a clinically defined condition. In the newborn, it consists of abnormal tone, altered alertness, possible seizures, as well as difficulty initiating and maintaining respirations.6-7 Hypoxic-ischemic injuries are the cause of a common subset of encephalopathy often referred to simply as HIE, but there are many other etiologies of encephalopathy.6-7
A sentinel event that indicates the time of injury can be determined in only about 25% of HIE cases.8 It is estimated that encephalopathy occurs in 2 to 5 of every 1000 term births/year, but the true incidence of HIE in the United States is unknown. As seen in Figure 1, between 7,900 and 19,750 of the 4,300,000 infants born in the United States in 2009 would have been potentially eligible for hypothermia based on the existing data.
Fig. 1. Estimated Incidence of HIE in United States

How Does Therapeutic Hypothermia Work?
In order to understand how treatment of the neonate with hypothermia results in the reduction of death and neurological impairment when measured later at 18 months of age, we must briefly examine the pathophysiology of HIE. The neurologic sequelae are the result of two distinct processes, primary and secondary energy failure, which are separated by a latent phase.7-8
Primary Energy Failure
Primary energy failure is the result of decreases in cerebral blood flow and oxygen delivery, which result in failure of aerobic metabolism. The resulting shift to anaerobic glycolysis is accompanied by excessive production of lactic acid, as well as tissue acidosis, depletion of the high energy phosphate compounds ATP and phosphocreatine, and inability to maintain cell membrane function. This results in loss of electrolyte gradients, with cell swelling and necrosis. The damage during this period occurs prior to hypothermia therapy and will not be affected by treatment.7-8
Secondary Energy Failure
After reperfusion of the brain, there is further evolution of cell death. During this phase of secondary energy failure the decline in phosphocreatine and ATP is not accompanied by brain acidosis, but it results in apoptosis, or programmed cell death. Apoptosis extends over several days, and the degree of energy failure and apoptosis is proportional to the severity of adverse neurodevelopmental outcomes seen later at one and four years of age. It is this phase of HIE that hypothermia attempts to ameliorate.7-8
The Latency Period
Fortunately, there is a brief recovery (latency) period between the two phases of injury that provides a therapeutic window for treatment. After reperfusion but before secondary energy failure there is a brief period following resuscitation when brain oxidative metabolism and cellular pH recover before the second phase of injury. Initial studies performed in animal models showed that hypothermia during this period was neuroprotective. It was concluded that cooling should be initiated as early as possible after the initial injury (preferably within 2, but no later than 6 hours) and should continue for 48 to 72 hours.8
Selective Head and Whole Body Cooling
The initial hypothermia trials with two separate devices were run concurrently (Fig. 2). One device, the Cool-cap (® Natus Medical Incorporated), was specifically designed for this purpose (Fig. 3) and is now approved by the FDA for hypothermia in newborns. [2] The other trialed device was a cooling blanket (Blanketrol ® Cincinnati Sub-Zero) (Fig. 4) that provides whole body cooling.1,3 This device was already approved for patient care by the FDA. During the Cool-cap trials, amplitude integrated EEG (aEEG) was performed as part of the inclusion criteria.9 Consequently, when the cap was approved as a medical device by the FDA, an abnormal aEEG tracing was required for its approved use. Thus, inclusion criteria for use of both devices is very similar with the exception of the aEEG requirement.
Fig. 2 Major Hypothermia Clinical Trials

Clinical Trials
Major clinical trials have examined the outcomes in both selective head cooling and whole body cooling. A 2010 meta-analysis of the three major trials examined the primary outcomes of death in 1320 infants and major neurodevelopmental disability after 18 months in 767 infants.5 There was a significant decrease in mortality/disability at 18 months (RR .81, 95% CI 0.71-0.93, p=.002). To prevent one infant death or one survivor with major disability, nine infants must be treated (95% CI 5-25).5 Thus far, the meta-analyses have shown no clear benefit of one method over another in outcomes,5 but trials continue and these results may change in the future.
Fig. 3. Infant on Selective Head Cooling using the Cool-cap(r) by Natus Medical Inc.

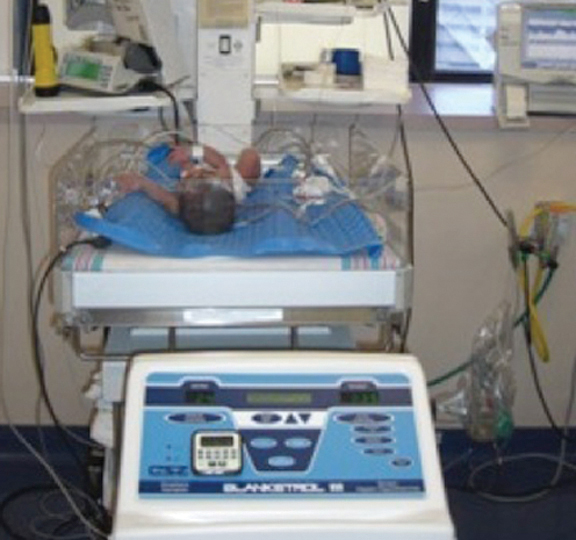
Fig. 4. Infant on Whole Body Colling using the Blanketrol III by Cincinnati Sub-zero.
Clinical Management
The inclusion criteria for both devices are listed in Table 1. If the child meets the initial criteria (items 1, 2, and 3 on Table 1), the child is placed on an aEEG. Figure 5 shows an example of a moderately abnormal tracing (bottom row) as compared to the normal tracing on the top row. If the child has a normal tracing and meets the initial criteria he/she may still qualify for whole body cooling. Our institution continues the aEEG throughout the 72 hour cooling process.
Table 1. Inclusion Criteria for Selective and Whole Body Cooling.

Table 2. Exclusion Criteria for Selective and Whole Body Cooling.

Fig. 5. Examples of aEEG tracings. Normal (top tracing) shows normal variability and sleep-wake cycle compared to moderately abnormal tracing on bottom.

Many of these children have systemic collapse associated with their HIE and close monitoring of injury to the heart, lungs, kidneys, and liver is critical. Ongoing laboratory measurements include blood gases, liver function tests, CPK, and electrolytes, as well as close monitoring of input and output. Good cardiac output and blood pressure are maintained to optimize brain perfusion. Acidosis and electrolyte imbalances are corrected, and normal glucose levels are preserved. Many of these infants require ventilator support as well as pressor support in order to achieve good oxygenation and perfusion. Several of our infants have required advanced respiratory support, including inhaled nitric oxide and extracorporeal membrane oxygenation (ECMO) for support of their pulmonary and cardiovascular systems.
Nursing care for hypothermia therapy includes close monitoring of neurologic status, and observation for clinical or sub-clinical seizures on aEEG. The nursing staff observes the infants for pain as well as any complications due to skin exposure to the cap or blanket. Close monitoring of vital signs, and assessment of respiratory and cardiac stability is key the care of these infants.
After 72 hours, the infant is warmed slowly. Careful attention is given to the aEEG at this time, because seizures may occur during rewarming. After the course of hypothermia, an MRI is obtained for structural assessment and a traditional EEG is used to examine function. During the recovery period occupational therapy and physical therapy are closely involved with families to help infants with residual feeding and tone problems. Once children are discharged, they are followed closely in neurodevelopmental follow up clinic. Our infants are followed for a minimum of 18 months, and preferably for three to four years. Many of these children are just approaching school age, and school performance will be a critical metric of this technique.
Conclusions
There is growing evidence that hypothermia can be used in the perinatal period to reduce cerebral injury and improve neurological outcome in infants who have HIE. Although the major clinical trials were performed well, unanswered questions remain. Unfortunately, only 25-35% of HIE cases have a clearly contributing sentinel event in the intrapartum period. In addition, a significant proportion of infants may have had chronic injury that began in utero plus additional acute injury at birth. These unknowns, as well as the extent of the initial injury, may contribute significantly to the variability in outcome following treatment. Additional unknowns include the optimum duration of cooling, the optimum rate of rewarming, and the safety and efficacy of hypothermia in premature infants. Continuing studies, as well ongoing neurodevelopmental evaluation of these children as they attend school, will begin to provide some of these answers. However, hypothermia is now considered a standard therapy and is no longer considered experimental treatment for HIE.
Resources
1. Azzopardi, D., et al., The TOBY Study. Whole body hypothermia for the treatment of perinatal asphyxial encephalopathy: a randomised controlled trial. BMC Pediatr, 2008. 8: p. 17.
2. Gluckman, P.D., et al., Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet, 2005. 365(9460): p. 663-70.
3. Shankaran, S., et al., Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med, 2005. 353(15): p. 1574-84.
4. Zhou, W.H., et al., Selective head cooling with mild systemic hypothermia after neonatal hypoxic-ischemic encephalopathy: a multicenter randomized controlled trial in China. J Pediatr, 2010. 157(3): p. 367-72, 372 e1-3.
5. Edwards, A.D., et al., Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ, 2010. 340: p. c363.
6. Cowan, F., et al., Origin and timing of brain lesions in term infants with neonatal encephalopathy. Lancet, 2003. 361(9359): p. 736-42.
7. Pfister, R.H. and R.F. Soll, Hypothermia for the treatment of infants with hypoxic-ischemic encephalopathy. J Perinatol, 2010. 30 Suppl: p. S82-7.
8. Higgins, R.D., et al., Hypothermia and perinatal asphyxia: executive summary of the National Institute of Child Health and Human Development workshop. J Pediatr, 2006. 148(2): p. 170-175.
9. Toet, M.C., L.G. van Rooij, and L.S. de Vries, The use of amplitude integrated electroencephalography for assessing neonatal neurologic injury. Clin Perinatol, 2008. 35(4): p. 665-78, v.